
Terry L. Rusch, et. al.
http://www.engnetbase.com
There are typically several measurement techniques that yield the same or similar data. Each technique will give information about different physical states, such as cardiac efficiency or tissue oxygenation state. For example, oxygen saturation and partial pressure of oxygen are related. However, the exact relationship is dependent on pH and temperature. A text by Kenneth McClatchey on Clinical Laboratory Medicine , the International Federation of Clinical Chemistry (IFCC), and instrument operator’s manuals are good references for laboratory protocols relating to invasively drawn samples [9,10]. Sample preparation and handling protocols, as well as standard measurement levels and physical states relating to excess or deficient readings are detailed. The range, price, and suppliers for instrumentation are extensive. A standard midrange laboratory blood gas analyzer can cost from $5,000 to $50,000. The more common measurements are described below.
The total concentration of hemoglobin (CtHb) or
hematocrit
(Hct) indicates the oxygen-carrying
potential of blood. The combination of amount of hemoglobin, partial pressure of oxygen or percent
oxygen saturation, and rate of flow of hemoglobin determines the amount and efficiency of tissue
oxygenation. Invasively drawn samples are typically used to measure hemoglobin and hematocrit values.
The total hemoglobin concentration is not the same as the red blood cell count, because red blood cells have different amounts of hemoglobin. The total hemoglobin concentration is measured optically by absorptive intensity at the isosbestic point. The Beers–Lambert law, also referred to as Beer’s law, shown in Equation 78.1 regulates the absorptive property of a substance.


Here the four most common Hb derivatives are used to calculate a more accurate CtHb. The individual
Hb derivatives are measured as outlined below.
Hematocrit is the volumetric fraction occupied by red blood cells and is generally measured by conductivity.
Hematocrit is also referred to as the packed cell volume (PVC). Hematocrit can be determined by
conductivity based on the plasma ion content. Hematocrit does not contribute to the conductivity and
therefore is inversely proportional to the conductivity. Hematocrit can also be determined optically in
various ways. One way is through optical density measurements, where the total optical density is the
sum of optical absorbance and optical scattering density. The total optical density is linearly proportional
to Hct, and, at clinically relevant Hct levels of 20 to 40%, scattering is dominant over absorption. One
optimization study showed that an optimum wavelength of 624 nm, at a measured angle of 90
¤
from the
incident light, gave an inverse linear intensity to the Hct level [11].
Oxygen tension or the partial pressure of oxygen (PO2 ) is a common measure of oxygenation states. The partial pressure of oxygen in hemoglobin determines how well oxygen is delivered to the cell tissue of the body. The more common partial pressure reference is arterial partial pressure of oxygen (PaO2 ). If the partial pressure of oxygen is higher than the surrounding tissue, oxygen is diffused to the tissue. If the partial pressure is lower than the tissue partial pressure, no oxygen is diffused to the tissue, and tissue damage can start to occur. Equation 78.3 shows Henry’s law.

C
is the concentration of oxygen (O2
),
Ls
is the solubility coefficient, and
PO2
is the partial pressure of
oxygen.
In diffusion, a partial pressure between the tissue and blood supply is trying to maintain equilibrium. Diffusion will occur until the two partial pressures are equal. Equation 78.4 shows diffusion equilibrium.

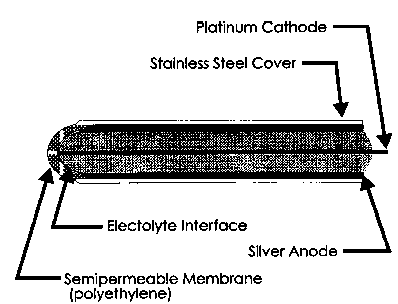
The rate of oxygen diffusion is dependent on the difference in partial pressure. The larger the difference
in partial pressure, the faster the diffusion rate.
The partial pressure difference is partially determined by the oxygen delivery (D0
) rate. Oxygen delivery
can be identified as the concentration of arterial oxygen minus the concentration of venus oxygen, times
the rate of blood flow (R), as shown in Equation 78.5.

CaO2
is the arterial oxygen concentration, and
CvO2
is the venus oxygen concentration. The oxygen
delivered, however, is not the oxygen delivered to the tissue. Some oxygen is transpired through the skin.
The transpired oxygen allows an alternative approximation measurement of arterial oxygen tension. The
more common oxygen tension measurement is arterial oxygen tension. Oxygen tension can be measured
electrochemically, transcutaneously, or optically. The techniques are listed below.