
Site navigation
• Curriculum vitaeRus • Ukr • Eng
• Abstract
Rus • Ukr • Eng
• Library
• Links
• Report about the search
• Individual task
• DonNTU
• Master's portal
SUMMARY
Theme of master's work:
Kinetic conformities to the law of synthesis of manganite lanthanum with the structure of perovskite on different technologies
Introducnion
In course of increasing development of microelectronics there was a problem of creation of systems with greater magnetoresistance. Such systems are necessary to create new high-sensitive magnetoresistance sensors for such important directions in modern technics, as emergency cuts-off of current in case of short circuit, for protection of people against defeat by a current, for protection of engines against damages. The devices which were applied earlier, became unsuitable because of their inertness. They could not provide fast enough switching-off of current.Lanthanum manganite perovskites containing strontium with exsess quantity of manganese (in the form of nanostructure clusters in a solid solution) are the most perspective materials for creation of sensors.
Now lanthanum manganite materials( AxB1-xMnO3; A – rare-earth metal B – alkaline-earth metal) represent the big theoretical and practical interest. Manganite compounds possessing strong magnetoresistance are widely demanded in applied science owing to their unique physical and chemical properties.
Numerous researches of lanthanum manganite systems basically concern physical aspects. Not enough attention has been paid to manufacturing techniques of powders and products from them, as well as to kinetic researches of synthesis.
The lanthanum manganite of following structure as object of research has been used:La0,7Sr0,3MnÎ3. This structure has been chosen due to the following reasons: 25 structures have been checked up, but today only the lanthanum manganite systems containing strontium, possess the optimal properties. Initial mixed material has been received by various methods: ceramic method (from oxides and carbonates) and chemical method (joint sedimentation and spray-type hydrolysis).
Method of experiment
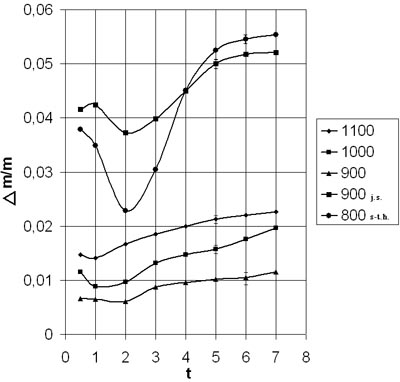
Considering that the most suitable method of synthesis of complex oxide systems is the ceramic method the majority of researches were carried out by this way. Results of researches were compared to data of the powders received by chemical technology.La2O3 (mark LaO – 1), SrCO3 and Mn3O4 have been used as raw material. Components were weighed on electronic weights with the maximum accuracy (6 figures after a comma). Then during 1,5 hours powders were mixed and regrated in an agate mortar for homogenization of structure and increasing activity of powders. Received mixed material has been placed in alumina bowls. Then it has been subjected to firing during various period of time (0.5, 1, 2, 3, 4, 5, 6 and 7 hours) at various temperatures (11000Ñ, 10000Ñ, 9000Ñ, 8000Ñ). Contents of bowls were weighed before and after firing. The samples were taken out at temperature of firing to carry out air hardening.
We have defined dependence of relative change of weight and phases structure at the given modes on temperature and time of endurance, in order to find optimum mode of synthesis of La0,7Sr0,3MnÎ3 with perovskite structure.
On the basis of researches diagrams of dependence Δm/m = f(τ) given in figure 1 have been constructed. Curves of firing of the powders received on ceramic and chemical (joint sedimentation and spray-type hydrolysis) methods, are represented on the diagram to compare them. Experiment was conducted by the same technology, as for powders received on ceramic method.
Conclusion
Results of researches of synthesis have shown, that using of chemical methods the temperature of firing is considerably lower than while using of a ceramic method.We offered ways of activation of process of solid-phase synthesis at the expense of briquetting and carrying out two- phases synthesis.
The received results make a physical and chemical basis of operated synthesis of the lanthanum manganite systems with perovskite structure and have significant practical value at introduction in manufacture.