www.fe.doe.gov/programs/powersystems/gasification/howgasificationworks.html
TRANSLETER
HOW COSL GASIFICATION POWER PLANTS WORK
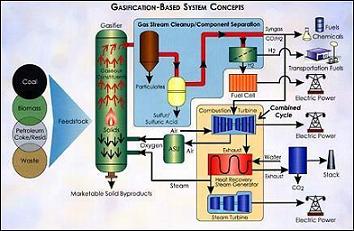
The heart of gasification-based systems is the gasifier. A gasifier converts hydrocarbon feedstock into gaseous components by applying heat under pressure in the presence of steam.
A gasifier differs from a combustor in that the amount of air or oxygen available inside the gasifier is carefully controlled so that only a relatively small portion of the fuel burns completely. This "partial oxidation" process provides the heat. Rather than burning, most of the carbon-containing feedstock is chemically broken apart by the gasifier's heat and pressure, setting into motion chemical reactions that produce "syngas." Syngas is primarily hydrogen, carbon monoxide and other gaseous constituents; the composition of which can vary depending upon the conditions in the gasifier and the type of feedstock.
Minerals in the fuel (i.e., the rocks, dirt and other impurities which don't gasify like carbon-based constituents) separate and leave the bottom of the gasifier either as an inert glass-like slag or other marketable solid products. Only a small fraction of the mineral matter is blown out of the gasifier as fly ash and requires removal downstream.
Sulfur impurities in the feedstock are converted to hydrogen sulfide and carbonyl sulfide, from which sulfur can be easily extracted, typically as elemental sulfur or sulfuric acid, both valuable byproducts. Nitrogen oxides, another potential pollutant, are not formed in the oxygen-deficient (reducing) environment of the gasifier; instead, ammonia is created by nitrogen-hydrogen reactions. The ammonia can be easily stripped out of the gas stream.
In Integrated Gasification Combined-Cycle (IGCC) systems, the syngas is cleaned of its hydrogen sulfide, ammonia and particulate matter and is burned as fuel in a combustion turbine (much like natural gas is burned in a turbine). The combustion turbine drives an electric generator. Hot air from the combustion turbine can be channeled back to the gasifier or the air separation unit, while exhaust heat from the combustion turbine is recovered and used to boil water, creating steam for a steam turbine-generator.
The use of these two types of turbines - a combustion turbine and a steam turbine - in combination, known as a "combined cycle," is one reason why gasification-based power systems can achieve unprecedented power generation efficiencies. Currently, commercially available gasification-based systems can operate at around 42% efficiencies; in the future, these systems may be able to achieve efficiencies approaching 60%. (A conventional coal-based boiler plant, by contrast, employs only a steam turbine-generator and is typically limited to 33-40% efficiencies.)
Higher efficiencies mean that less fuel is used to generate the rated power, resulting in better economics (which can mean lower costs to ratepayers) and the formation of fewer greenhouse gases (a 60%-efficient gasification power plant can cut the formation of carbon dioxide by 40% compared to a typical coal combustion plant).
All or part of the clean syngas can also be used in other ways:
• As chemical "building blocks" to produce a broad range of liquid or gaseous fuels and chemicals (using processes well established in today's chemical industry);
• As a fuel producer for highly efficient fuel cells (which run off the hydrogen made in a gasifier) or perhaps in the future, hydrogen turbines and fuel cell-turbine hybrid systems;
• As a source of hydrogen that can be separated from the gas stream and used as a fuel (for example, in President Bush's hydrogen-powered Freedom Car initiative) or as a feedstock for refineries (which use the hydrogen to upgrade petroleum products).
Another advantage of gasification-based energy systems is that when oxygen is used in the gasifier (rather than air), the carbon dioxide produced by the process is in a concentrated gas stream, making it easier and less expensive to separate and capture. Once the carbon dioxide is captured, it can be sequestered - that is, prevented from escaping to the atmosphere, where it could otherwise potentially contribute to the "greenhouse effect."
www.fe.doe.gov/programs/powersystems/gasification/howgasificationworks.html
