Investigation the structure and property of carbidosteel on base TiC, obtained by crucible melting method
Carbide-steel - a composition heat-strengthened material,
containing 30-35 % wt fine-dyspersated particles titanium of carbide or carbonitride,
which evenly portioned in matrix from tool steel,
heat-resistant steel, mainly from high-speed steel.
On the basis of cutting property carbidosteel borrow intermediate
position between high-speed steels higher of heat-resistance (R6M5K, R9M4K8)
and W-Co- alloys (the type VK8), exceeding last one on
transverse rupture strength on 600-800 MPa.
One of the varieties of carbidosteels possible to consider
the composition materials, which present itself metallic matrixes (the bases)
with given distribution in them reinforcers (for instance, disperse particles
and others.). At the same time individual properties of forming compositions
are effectively used. Combine volume content of components, it is possible,
depending on purposes, get the materials with require values of toughness,
high-temperature strength, module of elasticity, abrasive stability,
as well as create the compositions with necessary magnetic, dielectric,
radio absorbent and other special properties.
At present existing industrial methods of producing the carbidosteels
base on most cases on method of powder metallurgy with the following their pressing.
The aim of this investigation is a study of the possibility of obtain
carbidosteels of system Fe-TiC by method of the melting iron and carbide
of titanium in Tamman furnace.
Two samples of the alloy Fe-TiC with contents 25% TiC on mass (the sample 1)
and 16% TiC on mass (the sample 2) were melted in alundum crucible 30 mm
in diameter and height 80 mm under flux ANF-1P at the temperature 1700o Ñ.
The samples have made from obtained metal for fulfillment metallographic investigation. The samples were polished on abrasive tool, smoothed out on paper with different abrasive level and were polished on paste GOI and diamond paste. Then the sample was picklinged 10% solution HNO3 + ethyl alcohol. After that have carry out measure microhardness at load 100 gram.
Results of investigation of sample 1 has shown that it has a structure
hypereutectic white cast iron. Differs from usual white cast iron that
are present separate inclusion TiC (fig.1).
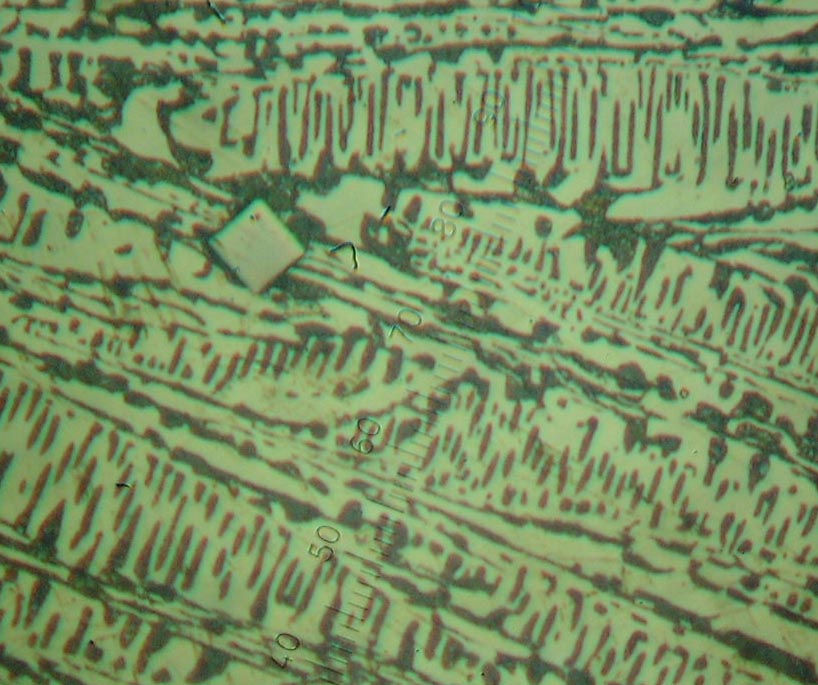
Figure 1 -Hypereutectic white cast iron
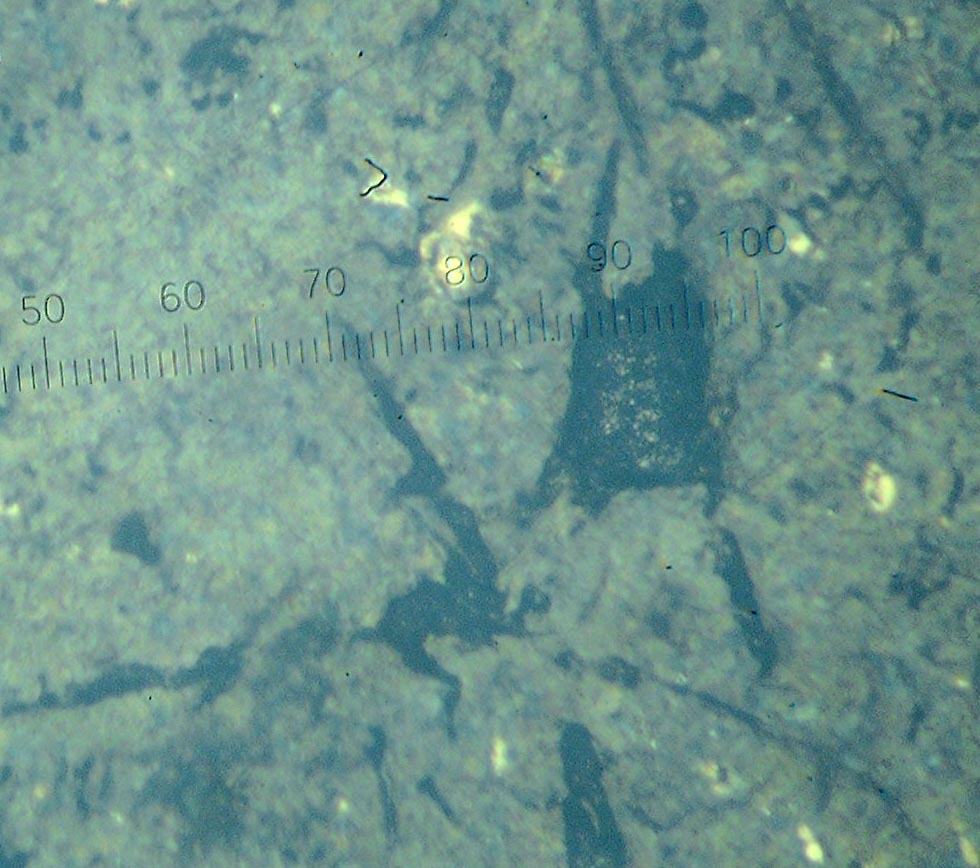
Figure 2-Carbidous matrix with separation of the graphite.
From figure 2 are seen that under smaller contents of the carbide full its dissolution has occurred, form the matrix of iron with separation of the graphite. In structure not pickled light area were revealed. Microhardness is above microhardness matrixes, but below microhardness carbide phase. Therefore for determination the nature of this area the additional studies are necessary to carry out. Technology of the melting brings about high dissolubility of carbide titanium in liquid phase and after crystallization quantity of carbide much small, that is in the following experiment it is necessary to enlarge the share of TiC.
In addition in samples microhardness was measured. Microhardness has measured in
white area, in dark area and in zone of the graphite.
Table 1- Microhardness of sample 1
| The white area |
The white band |
| N/mm2 |
N/mm2 |
| 7610 |
4450 |
| 7610 |
12870 |
| 5020 |
11660 |
| 5540 |
8570 |
| 6350 |
8940 |
| 5720 |
8940 |
| 5360 |
6570 |
| 5540 |
7060 |
| 5360 |
7610 |
| 4880 |
5540 |
| 4200 |
|
| Xcp =5744 N/mm2 |
Xcp=8221 N/mm2 |
| S2=(Xi-Xcp)2/n-1=1136447,6;
|
S2=6716765 |
The results of the measurement microhardness of sample 2 are provided in table 2.
Table 2- Microhardness of sample 2
| The white area |
Graphite |
The dark area |
| N/mm2 |
N/mm2 |
N/mm2 |
| 4450 |
4470 |
3220 |
| 5020 |
1730 |
1670 |
| 3970 |
1340 |
3970 |
| 3660 |
1130 |
4090 |
| 3570 |
1580 |
4200 |
| 5540 |
1210 |
3970 |
| 8940 |
2100 |
4330 |
| 9720 |
1730 |
5920 |
| 7060 |
1940 |
5360 |
| 8570 |
1080 |
8240 |
| 4880 |
|
|
| 5540 |
|
|
| 5720 |
|
|
| 4580 |
|
|
| 5540 |
|
|
| Xcp =5784 N/mm2; |
Xcp=1831 N/mm2; |
Xcp=4497 N/mm2; |
| S2=3739925; |
S2=979343; |
S2=3026623 |
Experimental is determined that solubility titanium of carbide in iron matrix above, than equilibrium, defined on diagram of state Fe-TiC. This can be connected with that that experiments carry out in graphite crucible, which created the condition for additional saturation of samples by carbon.


