 Figure 1 — In general, every color can be plotted three-dimensionally, and the color of a pigment is the result of the L, a and b values.
Figure 1 — In general, every color can be plotted three-dimensionally, and the color of a pigment is the result of the L, a and b values.Pigments can provide a full range of colors and are often the preferred coloring agent of end users because of their high thermal stability.
Ceramic pigments are substances that develop color in inorganic solids (ceramic or glass) and are capable of dispersing themselves at high processing temperatures without dissolution or chemical reaction. Pigments can provide a full range of colors and are often the preferred coloring agent of end users because of their high thermal stability.
Pigments are used in a variety of applications, including as coatings for ceramic and glass, and in plastics and inks. The decorating industry uses pigments combined with suitable fluxes in on- and under-glaze applications.
Understanding Color
Isaac Newton made a study of color and developed the useful Newton color circle, which gives insight about complementary colors and additive color mixing. Newton took the bar of colors created by the passage of light through a prism and transformed it into a segmented circle, where the size of each segment differed according to his calculations of its wavelength and corresponding width in the spectrum. Newton's Theory of Color states that objects appear to be certain colors because they absorb and reflect different amounts and wavelengths of light.
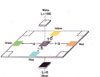
In general, every color can be plotted three-dimensionally through its brightness ("L") and shades ("a" and "b" indicate the position on a two-color axis, with "a" on the red-green axis and "b" on the yellow-blue axis). The brightness axis runs perpendicular to the color panel, with L = 100 for white and L = 0 for black. The color of a pigment is the result of the combined L, a and b values, which form the color space [2] (see Figure 1).
 Figure 1 — In general, every color can be plotted three-dimensionally, and the color of a pigment is the result of the L, a and b values.
Figure 1 — In general, every color can be plotted three-dimensionally, and the color of a pigment is the result of the L, a and b values.CIE L*a*b* (CIELAB) is the most complete color model and is used conventionally to describe all of the colors that are visible to the human eye. It was developed for this specific purpose by the International Commission on Illumination (Commission Internationale d'Eclairage) [2].
Richard Hunter created a new tri-stimulus color model called L*a*b*. He scaled color space in an effort to achieve the uniform spacing of colors, thus making the evaluation of total color deviations possible. Deviations, or the total color differences compared to a given target color, are expressed as ?E and can be calculated according the following formula:
E = v(L2+a2+b2)
The L value measures intensity; as the L value increases, the sample becomes lighter until it reaches white (L = 100). A positive "a" value represents increasing red, while a negative "a" value represents increasing green. Likewise, a positive "b" value represents increasing yellow, and a negative "b" value represents increasing blue.
The production of ceramic and glass articles is an ancient art. We know from Chinese and Egyptian potteries dating from 3000-1000 B.C. that natural minerals containing cobalt (Co), chromium (Cr), iron (Fe) and manganese (Mn) were used as coloring agents. Later, Chinese potters introduced red cuprous oxide, yellow lead antimonite, blue cobalt silicate, and brown manganese silicate [3].
Before the industrial revolution, many pigments were known by the location where they were produced, and pigments based on minerals and clays often bore the name of the city or region where they were mined. For example, raw sienna and burnt sienna came from Siena, Italy, while raw umber and burnt umber came from Umbria in central Italy.
The industrial and scientific revolutions brought a huge expansion in the range of synthetic pigments, which are pigments that are manufactured or refined from naturally occurring materials. Color manufacturers started investigating new techniques and modern manufacturing processes to develop stronger pigments [4]. Most of these pigments were also used as coloring agents in the iron, steel and aluminum industries.
The control of raw materials is a must for the production of today's high-quality pigments. Some pigments require the use/addition of synthetic materials in order to guarantee the purity and strength of the color. Raw materials are weighed according to a given recipe and blended thoroughly. The blend is then charged into saggers and calcined to a given temperature until the reaction process is complete. Both atmosphere and temperature are monitored with the help of control equipment.
The calcined pigment is then milled to a given particle size in a ball mill or pulverizer. The next stage in the production process is to remove any soluble salts present in the pigment using a filter press or by washing the material in a tank. The final dried pigment is tested for quality, particle size, color tolerance and stability according to given specifications.
With the development of a modern color industry, manufacturers and professionals have cooperated to create international standards for identifying, producing, measuring and testing colors. The chemical classification of pigments is based on the content of the main coloring elements, such as Fe, Cr, Mn, Co or Cu.
This classification system is not adequate, however, as pigments with different color characteristics may fall into one group. The field of colorant classification has been well defined by the Dry Color Manufacturers Association (DCMA), which bases the classification on colors5.
Crystal chemistry is the basis for understanding the structure of ceramic pigments and their classification into different groups. Modern analytical techniques like X-ray analysis are used to deepen the knowledge of pigment structures and help developers to not only control the properties of existing pigments but to produce new pigments as well.
"Color Wheel," en.wikipedia.org/wiki/Color_wheel.
Viswanath, A., "Possibilities of Color Development," presented at the State College of Ceramics-Germany, January 31, 1991.
Kharashvili, E., "Trends in Developing Ceramic Pigments," Steklo I Keramica, Vol. 10, pp. 20-22, 1985.
Bell, Bernard, The Development of Colorants: Review of Progress in Coloration, Vol. 9, p. 48, 1978.
Classification and Chemical Description of Colored Pigments, 2nd Edition, Dry Color Manufacturers Association, January 1982.