
 DonNTU |
Master's
portal
DonNTU |
Master's
portal

| Resume | Biography |
The world's population is growing steadily. Demographic growth is accompanied by a growth of consumption of resources such as food, mineral and energy resources. Food chemists and biochemists are successfully coping with creation of new types of crops and synthetic products. With natural resources, especially energy, everything is much more complicated. Over the past half century has greatly increased production and consumption of nonrenewable energy efficient resources: oil, gas and coal. At current rate of consumption, in several hundred years, there may come a time when the burn will be nothing. Most people either do not think about it or believe that will be opened a new absolute source of energy. But until that happens, we should also think about rationalization of consumption, more efficient ways of processing and extraction of raw materials, and about new features of energy use of carbon materials. In my opinion, the direct coal fuel cell (DCFC) is promising technology of improvement production of energy from coal.
The search for new sources of more efficient energy use always been and remains one of profiling direction for entire scientific community. At the moment, electricity from coal is receiving by burning it for getting steam.
The possibility of direct conversion of energy ties in coal into electrical energy is potentially more effective, as efficiency increase while reducing mechanical losses and heat loss.
It is necessary to invent new types of batteries of local application using cheaper raw materials. Using of interchangeable domestic energy carriers will significantly reduce the cost of energy for consumers.
I. Overview of research on this subject
A fuel cell is an electrochemical cell that converts chemical energy from a fuel into electric energy [1]. At the moment, the world-known and widely used hydrogen fuel cell (Fig.1).
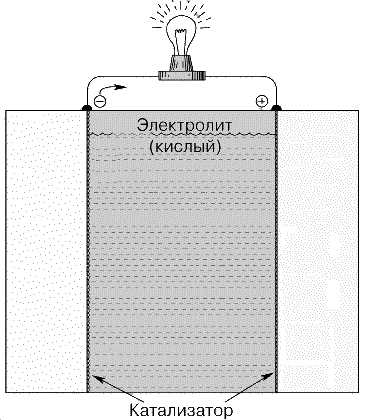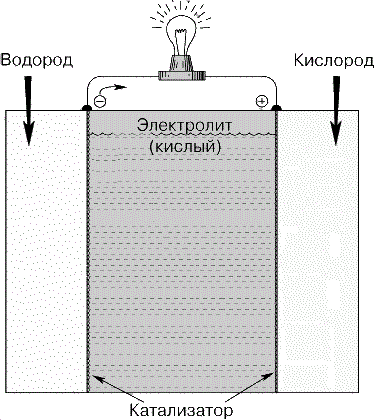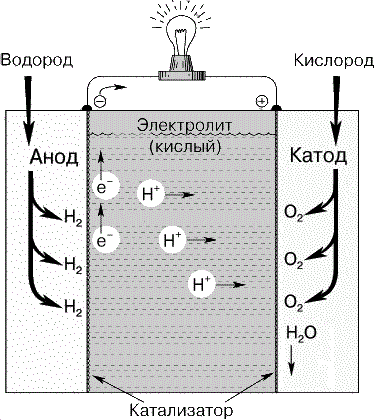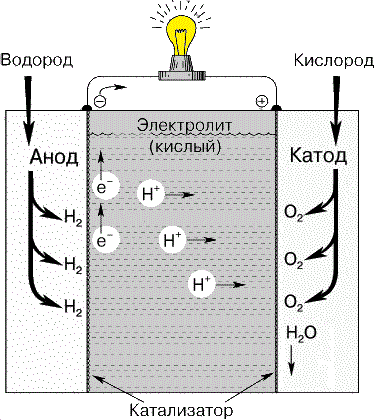
Рисунок 1 – Водородно-кислородный топливный элемент
The famous German chemist Wilhelm Ostwald was convinced that fuel cells have an advantage over all known sources of energy, which today is completely confirmed.
Burning atoms of fuel lose electrons and the atoms of oxygen get them. In oxidation process atoms of carbon and oxygen are joining in the products of combustion - carbon dioxide molecules. This process is energetically: the atoms and molecules involved in burning get great speed, and this leads to an increasing of temperature.
The chemical reaction of carbon combustion is as follows:
C + O2 = CO2 + heat
In the process of burning chemical energy convert’s into heat energy through the exchange of electrons between atoms of the fuel and oxidizer. This exchange occurs randomly [2].
Carbon fuel cell consists of anode, cathode and electrolyte (Fig. 2). At the anode the reductant oxidizes. The free electrons from the anode enter the external circuit, and positive ions hold at the anode-electrolyte boundary. On the other end of the circuit the electrons move to cathode, on which reduction reaction is going [2]. Then ions of oxidant transfer throw the electrolyte to the cathode.
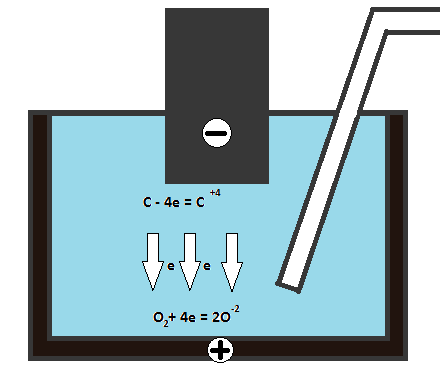
Figure 2 - The unit of coal fuel cell
These elements were invented by William Jaco and patented in the USA in 1896, patent number 555 511 [3]. The inventor has built a capacity of up to 2 HP and they were periodically tested for several months. The power unit of the elements showed a coefficient of performance (COP) to 35%, which is a very high value of the efficiency of energy even today. However, his opponents allegedly proved that in his element there is no burning of coal, but there is only a thermoelectric generation with efficiency of only 8%. After that, his invention was forgotten until 1973, and was not mentioned in books and publications. In the early 70's research group in the United States repeated the experiments of Jaco, and became convinced that Jacko really has created a fuel cell [4]. [6].
The English-language resource contains numerous information sources of this question. Especially in this issue were succeeded two American companies: "SRI International" and "SARA".
SRI International is one of the world's leading independent scientific research centers, located in Silicon Valley. In 2005 at a private seminar devoted the direct conversion of carbon, the chief engineer Yuri Balachov and vice president Lawrence Dyuboy introduced their technology of coal fuel cell. According to them, their device can operate on coal, coke, fuel oil, biomass and organic waste. Produced electricity is 2 times cheaper than coal-fired power plants. Waste gas is almost entirely composed of pure CO2 [5].
Scientific Applications & Research Associates (SARA) - joint-stock company founded in 1989, engaged in scientific development by government and private orders. This company has been developing on this issue since the mid-90s and is currently provided by the third generation of its direct coal fuel cells, which reached 60% efficiency and average output power of 16 watts for 540 hours [6].
In Ukraine, the development of the coal fuel cell is much smaller than in the U.S. and Japan. Among the Ukrainian scientists it can be distinguished a member of the National Academy of Sciences of Ukraine Kovtun G. and Candidate of Chemical Sciences Polunkin E. that deal with alternative energy sources, Doctor of Physical and Mathematical Sciences Vasiliev A., which deals with the problems of hydrogen energy.
Within DonNTU Master of Physical and Metallurgical Faculty Dolzhikova Elena and her supervisor Pyatyshkin G. were dealt with this problem.
II. The experiment and findings
To use any of the coal fuel cell it is necessary to have following components:
In this unit is combined the anode and fuel, using a graphite electrode. Graphite rod was taken because of its excellent electrical conductivity. This allowed us to do not think about the quality of used fuel and focus on the optimization settings.
The stainless steel tank acts as a cathode. Stainless steel is an excellent conductor and it is practically immune to the corrosive effect of the electrolyte.
Sodium hydroxide melt acts as electrolyte.
Supply the oxidation zone by oxygen is performed by feeding air through the iron tube. Air injection performs by the compressor.
Schematic diagram of the unit is shown in Figure 3.
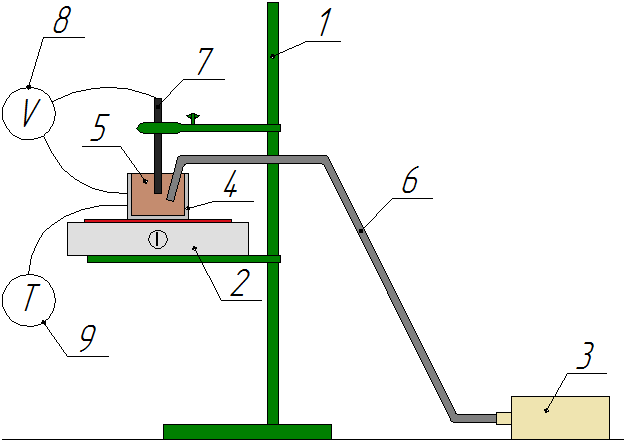
Figure 3 - Schematic diagram of the unit: 1 - tripod, 2 - electric
stove, 3 - compressor,
4 - stainless steel tank (the cathode), 5 - electrolyte, 6 - air tube,
7 - graphite electrode (the anode), 8 –voltmeter, 9 -
thermometer with a thermocouple
The electric stove should heat up to 400-500 º C. Compressor power and the diameter of the air tube should provide optimal sparging without splashing.
It is necessary to conduct the experiment under a good ventilation to avoid contact of alkali vapors with the respiratory tract. It is necessary to protect the skin, eyes and clothing from splashed molten sodium hydroxide.
The procedure of the experiment:
For the experiment were taken following design materials and substances:
Results:
III. The calculation of the efficiency of the coal fuel cell
The calculation of the real efficiency will be carried out according to the method proposed by the Russian Federal Nuclear Center [7].
The reaction occurs according to the equation:

Determine the thermodynamic (ideal) and real efficiency for DCFC.
For the fuel cell the ideal efficiency (ηi) is represented as

where n
- number of electrons involved in the reaction; F - Faraday constant;
ΔH - enthalpy of reaction; Em - the average difference
between the equilibrium electrode potentials of the element with the
full use of fuel or electromotive force:

Then

The real efficiency (ηr) is defined as

where
ηF - Faraday efficiency, ηe - electrical efficiency.
Under the Faraday efficiency of fuel cells means the ratio of electricity actually obtained in the fuel cell from one mole of reducing agent (qr) to the theoretical amount of electricity (qt).
Real amount of electricity:

where I
– amperage, t – time of work, ν –
amount of substance graphite.

Theoretical amount of electricity may be founded as


Then the Faraday efficiency is

Electrical efficiency is defined as

where U
– cell voltage.

Thus, the actual efficiency will be:

This is value for a single non-optimized direct coal fuel cell. Practical power to fuel cells according to preliminary calculations can reach the value of efficiency at 70-75%, which is 1.5-2 times greater than thermal efficiency of coal power plants.
Analyzing the first calculations its can be said that the idea of generating electricity from the fuel in the fuel cell, avoiding burning, has the right to exist. Among the goals of the work on this project should include the following:
Carbon fuel cell is an environmentally friendly source of energy: high efficiency will reduce consumption of fuel resources. Only CO2 releases to the atmosphere during the reaction, sulfur oxides and all other elements is connected with alkali.
| Resume | Bigraphy |