Introduction (Motivation)
Actuality theme is that the electroslag technology in a graphite crucible is very promising. This is due to "mobility" crucibles, because they can melt a small mass of metal, graphite does not react with copper, therefore, is an excellent material for the lining. This allows us to produce high-quality metal in small quantities that satisfy the needs of small businesses.
Scientific meaningfulness of work: in the long run to choose the most versatile of the slag and Optimal conditions for the ESR bronzes.
Practical value the work is that it allows you to organize small production directly in the shop. Material for the melting may be sourced from scrap business. This may solve the problem of disposing of scrap and improve the economic efficiency of the enterprise.
Abstract
Melting of cast bronze makes use of various types of furnaces. Each type has its advantages and disadvantages. Feature of the arc furnace are increased waste metal, its gas saturation. In coreless induction furnaces low productivity, low thermal efficiency, increased power consumption. For channel induction furnaces need to have a constant level of so-called "swamp", which creates difficulties when switching from one brand to another alloy. For reverberatory furnaces is characterized by high consumption of fuel, most of the complexity of services, significant oxidation of the metal during smelting. Vacuum furnaces are complex and bulky equipment. A characteristic feature of the shaft furnace is the difficulty of adjustment, large coke consumption, and poor quality metal.
Many of the shortcomings of these methods eliminated the use of electro slag technologies, in particular electro slag crucible melting (ESHTP). The process of electro slag remelting in a graphite crucible - a special method of refining, in which weld consumable electrode is melted in a layer of molten slag, or in the case of small-sized batches (eg, chips) remelting are non-expendable graphite electrode. Changing the composition of the slag can be used in a wide range to adjust the temperature of the melt and, consequently, intoxication and low-melting metals (eg zinc in brass or tin, tin bronze), with remelting of copper-based alloys, there is no interaction with the carbon lining, so the melt is not contaminated with crucible material, high thermal conductivity and low temperature coefficient of linear expansion of graphite cause high resistance and durability of the crucibles, because of the easy workability of graphite; crucibles cost less than other materials.
The aim of this work is to choose the optimum composition for the slag remelting of secondary bronzes. On the one hand has to ensure the stability of slag remelting process, which determines its electrical resistance, and, on the other hand, it must ensure that the desired chemical composition of the remelted metal and reduce carbon monoxide and the oxidation of the alloy. Based on the tasks that reduce factor to the refining of metal oxides and protect it from oxidation, a key requirement for slag ESHVR expressed that they should actively dissolve the formed oxides.
The development of slag is carried out in several stages: the melting temperature is determined by the selected slag ¬ composition, its viscosity is qualitatively assessed in the operating temperature range and speed of immersion in a lightweight blend melt. Metal and slag are crystallized in the crucible. The mass of metal and slag before and after melting, as well as their chemical composition is judged on the heat of the metal, alloying elements, the loss of slag components and interactions between the metal and slag, which establishes the line- is called the selected conditions of electro slag, slag process, mainly electrical. In particular the stability of the regime and composition. If necessary, adjust the composition, introducing the additives of oxides or salts. Investigate the viscosity and conductivity of the slag melts in the operating temperature range and the effects of corrective agents. From the practice of ESR it is known that such a universal solvents are halides of alkali and alkaline earth metal. As the slag in the ESR is used salt, oxide, and combined with salt-oxide slag. Due to the large solubility of hydrogen in copper alloys and the possibility of oxidation of the alloy oxide and the combined use of slag is not desirable. In connection with the foregoing, the ESR of copper alloys as slag can be used the following components: CaF2, CaCl2, NaF, and their composition. Calcium fluoride (CaF2), having a melting point 1411°C, considerably cheaper than the other fluorides and has the lowest vapor pressure. Therefore, it is widely used in the ESR in pure form or as a main component of a complex salt slags. However, for copper and copper alloys, it is unacceptable because of its high melting point. Lower melting temperature (650°C) and the cost has CaCl2, but it is strongly hygroscopic, it complicates the storage and contaminates the metal with hydrogen. Sodium fluoride (NaF) (melting point of 997°C) has a high vapor pressure, which leads to copious fumes in the process of refining, and it is very toxic.
Reduce the melting temperature of the slag can be used through the use of two-component salt slags based on CaF2: CaF2-CaCl2; CaF2-NaF. They are in a fairly wide range of concentrations having melting temperatures below the casting of most copper alloys. In addition, the melt have a wide range of crystallization, which facilitates the melting of the slag bath at a "solid state". So, if you use Cacl2 already at 650 ° C the appearance in to the liquid phase. These systems as a whole satisfies the requirements satisfy the requirements for dissolution of oxides. They give a homogeneous melt with oxides CaO, SiO, ZnO, TiO2, Cr2O3, Al2O3, in concentrations of the last 5 to 25% depending on the si tem and dissolved to 1% Cu2O. Regarding shortage of components and cost of these systems is almost uniformly valuable. It is worth saying about such indicators as the stability of the composition. In general, all of the slag showed satisfactory stability of the composition. General trends dency to change the composition of the following. As a result, apparently, electrolytic, and possibly heat, dissociation is a decrease in the fluorine content, and increased calcium content. These changes were more pronounced the higher the temperature of the process. They may be associated with the formation of free elements. In addition, when, smelting aluminum bronzes pointed out that aluminum reacts with the substitution of calcium fluoride, sodium fluoride and restores the silicon from silica slag, which goes into the metal. As a result of simultaneous action of several factors - AlF3 ash into the atmosphere, reducing the amount of fusible components and improve the content of refractory (CaO Al2O3) slags lost their properties and stability are impaired. These changes occurred most intensively during melting chips.
The table presents data on the composition of slag for certain intervals of work, ie, of finding it in a molten state during smelting and refining of metal. From the data presented the slag can long retain its membership in smelting copper and brass. Smelting of aluminum bronze composition for the above reasons vary quite markedly. Under ESHR and ESHVR by the irreversibility of the reaction between carbon and copper oxide produces the same deep deoxidation of copper, as well as in vacuum (induction, electron beam) melting. In addition, the oxides of copper do not accumulate in the slag, which provides a constant refining this ability and negligible loss of metal. As a result, a small amount of slag can affect large masses of metal. Should focus on reduction of oxides of zinc (Fig. 1). When adding graphite shavings, restoration of zinc is accompanied by intense burning, resulting in the total amount recovered only to about 10% goes into the metal, and the rest in the form of dust goes into the vent pipe.
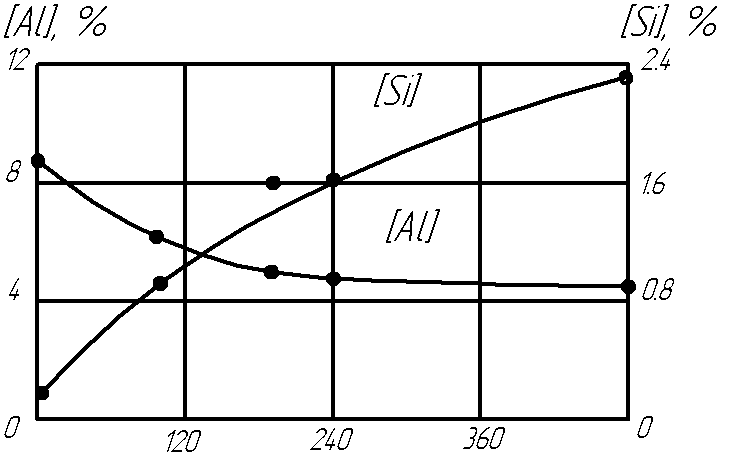
Figure 1. Dependence of maintenance of aluminium and silicon at ESR of bronze of LZH 9-4 from self-control
Without the addition of a reducing agent combustion of zinc is also observed, but the process is fairly easy. The share of zinc pereshed-Sheha in the metal increases by about 50%.Oxides of aluminum in the slag ESHVR not appreciably recovered we establish. On the contrary, aluminum metal (bronze AF 9 - 4) is oxidized in the presence of slag SiO2.The content of silicon in the metal rises and falls of aluminum. Just not appreciably recovered we establish SiO2 contains of most toxins. And the real conditions during melting of multi component alloy under the direction of multi-component slag oxi ¬ oxidative - reduction processes in the metal and slag will obviously be determined by the selected potential of this particular reaction, or, in other words, the affinity of the elements for oxygen. However, the correct choice of the slag such side processes can be completely excluded. Measure compliance with the slag are the stability of its composition and preservation of the metal. Sources of hydrogen ESHVR copper and its alloys can be, on the one hand, charge materials (copper cathode, shaving with the remnants of the emulsion), on the other - the atmosphere furnace. This hydrogen is in a deep deoxidation of metal and can cause porosity of ingots and castings.
Transition of hydrogen in the metal can impede and delay, by varying the composition of the slag, slag pool depth and character of the furnace atmosphere. So, when melting aluminum bronze in open furnaces tend to be on the surface of metal oxide thin film, as in a furnace to maintain an oxidizing atmosphere. Under these conditions, the oxide film hinders the diffusion of hydrogen.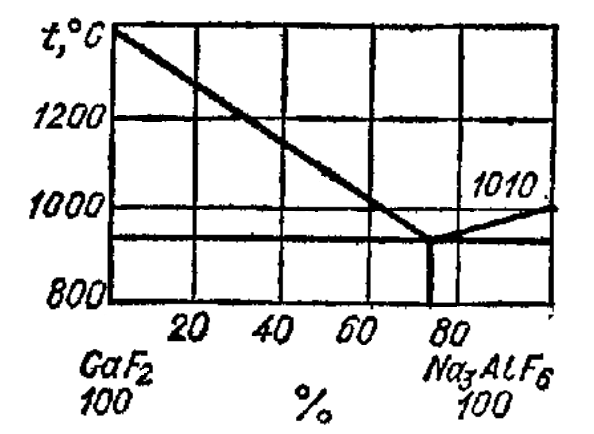
Figure 2 - Chart melting of CaF2 and Na3AlF6
For the protection of the metal from being saturated with hydrogen at ESHVR can recommend, first, by accelerating the melting reap the holding time of metal under the slag and, secondly, to maintain a minimum concentration of moisture in the atmosphere furnace and, thirdly, to use a metal charge and slag forming components in the calcined to remove moisture condition.
It should stay on the choice of slag and its chemical composition. Slag CaF2 - NaF is preferred (does not saturate the metal with hydrogen), and its toxicity can be reduced considerably by replacing NaF in its composition, for example, Na3AlF6 (cryolite). Based on the phase diagram (Fig. 2) to the desired melting point (≈ 900°C) for melting of copper alloys has slag close to the eutectic composition: 80% CaF2 + 20% cryolite.
| Material | Chemical composition, wt. % | |||||||
|---|---|---|---|---|---|---|---|---|
| Sn | Zn | Pb | Sb | Fe | Al | Si | Cu | |
| Initial charge:Br. OTSS6-6-3 | 6.85 | 6.93 | 2.71 | 0.5 | 0.4 | 0.05 | 0.05 | rest |
| Alloy after remelting | 6.67 | 3,25 | 2.58 | 0.4 | 0.45 | 0.05 | 0.05 | rest |
