
Faculty: Physical-metallurgical
Speciality: Industrial hittechnics

Fiery furnaces are widely spread in various industries. These ovens are heated by a liquid and gaseous fuels. Among the various types of gaseous fuels first place belongs to the natural gas application of which is continuously expanding. Natural gas provides the heat of combustion, and contains a large methane, which in case of need to get a luminous flame.
radiation heat transfer in the working space of high-temperature furnaces is the predominant heat transfer depends both on temperature and on its emissivity. As is known, the luminous flame is different from non-luminous so that it radiates heat energy is not selectively, within the bands of CO2 and H2O, and across the spectrum, including the visible region. And it is in the visible region of the luminous flame of natural gas emitted significant amount of heat energy. This is explained by the fact that the dispersed phase (soot), which arose in the expansion of mental, contains in its composition the particles of this size (0,05-0,2 mm) that show maximum radiation is in the visible and near-infrared spectrum. Thus, the emissivity of luminous flames are significantly higher than emissivity of the non-luminous flame. This ability luminous flame is the reason for its use in practice.
In some cases, conditions of work are furnaces, which intensify the heat transfer by radiation as a result of increase in temperature or not (limited by the resistance of refractory materials), or irrational. In such cases, an increase heat flow can be ensured by increasing the emissivity of the flame, ie, increase in its luminosity. In other cases it is advisable to use both of these possibilities (and the temperature and luminosity) to enhance heat transfer from the flame.
is well known that the luminosity of the flame does not arise spontaneously, that natural gas can produce and non-luminous flame. To create a luminous flame to the combustion of natural gas to create such conditions under which will obespechitvatsya pyrolysis of methane with the emergence of a new dispersed phase - black. However, depending on the conditions of (temperature and time of the process, the oxidation of the gas phase) dispersed phase will have different characteristics: different size and concentration of smoke particles. Since the change in particle size and concentration has a significant influence on flame radiation, a process pyrolysis should be conducted under optimal boundaries. It is these optimal conditions for the expansion should be ensured those methods that are used for natural gas combustion in practice.
According to our calculations and heat balance, and draw conclusions about the irrational method of burning fuel. Ways to reduce fuel consumption at the same performance of 4 t/h synthetic slag and were described in this paper.
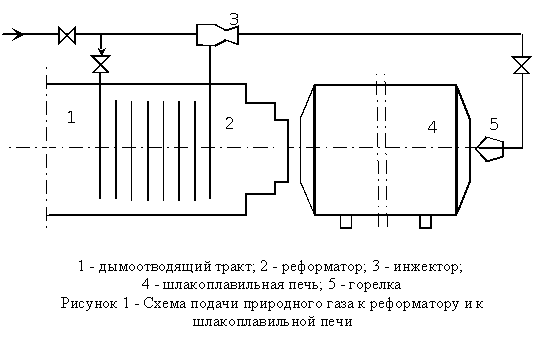
To improve heat and mass transfer processes, the furnace, it is necessary to increase the radiation characteristics torch in its operating space of the furnace. One way to increase the luminosity of the torch a heating furnace reformed natural gas. To this end, part of the natural gas from the total amount going to the manufacturing process, is sent to a special unit - the reformer, installed in the exhaust tract of the furnace. At high temperature (1100 - 1300 ° C) methane-intensive decomposed into carbon black carbon and hydrogen, and resulting mixture is fed into a gas burner stove. The presence of a gaseous fuel a large number of soot particles with preferred sizes from 0, 2 to 0.5 microns dramatically increases the luminosity of the torch and leads to an increase in heat transfer by radiation. The principle of operation of the reformer is the following: natural gas pipeline from the total allocated into two streams: minority (10% of the above calculation) is sent to the steel tubes of the reformer, made heat-resistant steel, while the bulk of the gas supplied to the injector, through which compensated pressure drop for gas flow through the tubes of the reformer.
GENERAL DESCRIPTION OF LUMINOUS FLAME NATURAL GAS
1. Natural gas and its properties In a large natural gas calorific value, which provides the calorimetric combustion temperature more than 200 0 C, and allows its use as a fuel for high temperature furnaces. The lower limit of ignition of natural gas (nepodogretyh mixtures) is 5,34-5,41%, the upper limit 14,39-14,50%. Maximum flame speed 0,68 m / sekdostigaetsya for the content of the gas in mixture with air 9,45-9,77%. Natural gas is colorless, it has no smell.
2. Luminous flame, and its application To create a luminous flame, you need to create conditions under which happens decomposition of hydrocarbons fuel to form a new dispersed phase (soot), which has rational characteristics.
There are basically two ways of creating such an environment:
1. Preliminary (before the release of a working space of the furnace), the expansion of natural gas.
2. Decomposition of natural gas directly in the space of the furnace. The basis of the decomposition process is the same and consists in heating the gas to the required temperature during certain time. The difference between these processes as follows:
1) In the first case, the expansion of natural gas is usually carried out in the process of oxidative pyrolysis ie in such circumstances, when a part is burned, and another part is heated to a certain temperature, the heat which is released during oxidation.
2) In the second case the gas is fed to one (or more) jet in the workspace and heated as a result of heat absorption, which is transmitted elements of the workspace. In this case, the gas supply should be implemented in such a way that heating or expansion of gas (or part thereof) have been implemented earlier than its complete oxidation. In practice, deliberately delaying mixing of gas and air. After the appearance of a dispersed phase of its further transformations depend on many factors, of which almost the main thing is the size of its particles. The smaller the particle, the faster it is oxidized and graphitized, the shorter will be illuminated part of the flame. Comparing these two paths should be preferred to the second, as more straightforward. In fact, it does not have nor create any additional devices to be placed outside the furnace or furnace rebuild. However, this apparent simplicity and deceptive. The fact that the process of pre-reform, in principle, easier, and because better studied and more unmanageable. A reform process in the jet is extremely difficult, practically not been studied, and therefore virtually unmanageable. This is explained by the fact that the flame represents a set of complex occurring almost simultaneously processes. These include physical and chemical processes. The former include aerodynamic and heat exchange processes, the latter - the processes of combustion, gasification, pyrolysis. In the flame, these processes are often inseparable, therefore the study of the flame is usually carried out by one of two directions, which can be defined as scientific and industrial.
extreme difficulty of establishing well-luminous flame in the open-hearth furnaces, it is difficult workable in practice very narrow optimum temperature-time interval of decomposition of methane - all determined to have a desire for stoves special device - a reformer, which is placed outside the oven and allow to reliably manage the required reforms quantities of natural gas. The principle of operation of the reformer the next. In the first (lower) the burner is fed gas (or gas and fuel oil), which reformed interacting with heated air in the regenerator. The heated air is drawn into the reformer as a result of injecting of the jet of the second (top) burner, which serves about 60% of the gas, the main oil and compressed air. The Reformers supported the coefficient of oxygen 0,2-0,25 that in the presence of heated air can do without additional heating chamber reformation. The residence time of gas in the reformer does not exceed 0,6-0,7 sec. The reform process at a temperature of 1050-1150S. The yield of soot is 90-120 g / m 3. The use of such Reformation devices on furnaces, which smelt stainless steel, with natural gas and sulfur fuel oil has given good results. Performance furnace increased by 5 - 7%, the specific fuel consumption decreased by 4.5 - 7%, stability of the main dome and nozzle regenerators increased by 12 - 15%.
3. Influence of degradation products of natural gas on the emission of the flame
Under certain conditions, the expansion of natural gas (methane) in decomposition products may the simultaneous presence of carbon black as carbon, as well as intermediate products of decomposition, which may have different effects on the radiative properties of flames.
Scientific Botcher conducted pre-heating gas in a special heater and its subsequent burning and received, the maximum emission was observed at 1000 - 1150 C. Moreover, at higher temperatures heater flame radiation decreased. This same temperature range corresponded to the most liquid hydrocarbon compounds produced by decomposition of methane.
Similar findings have Rummel and Vee, and they felt that the greatest influence on the emission of the flame provide intermediate compounds formed during the decomposition of methane. The mechanism of this effect appeared to authors as follows: in the process degidrogenezatsionnoy condensation and oxidation of hydrocarbon compounds released atomic hydrogen-burning near the surface of the particles gradually enriched with carbon. This leads to an increase the surface temperature of the particles and ultimately - to the more intense radiation. Liquid intermediate decomposition products (Tars) are formed only in a certain temperature range. Therefore, the increase in flame radiation preheating the gas to the same temperature is quite natural to associate it with the presence of intermediate decomposition products.
But Shaq, to ??perform the analysis of heat transfer smoke particles from the surrounding gas phase, showed that the difference temperature gas flow and particle does not exceed 1 - 2 deg. This fact has called into question the hypothetical assumption Rummel and RE on the causes of significant influence of intermediate products of decomposition of methane on the flame radiation. Must be borne in mind that the increase in decomposition temperature from 1050 to 1200 ? C results in an increase of hydrogen content in the dispersed phase with a simultaneous increase in the diameter of the particles. This is a very important fact, since it increases the diameter of the particles may be the reason that in the region of maximum formation of intermediate degradation products (namely, they contain hydrogen) is the emissivity of the flame is maximal.
EXPANSION OF NATURAL GAS
1. General characteristics of the pyrolysis of methane and the formation of the dispersed phase
Methane is the foundation of natural gas, so the pattern of the pyrolysis of methane are determinants and the process of decomposition of natural gas. In practical terms reforming of natural gas is carried out to isolate pyrolysis of methane of the dispersed phase, which greatly increases the emissivity of the flame.
Thus, the dispersed phase suspended in the gas phase, is the product for which hold decomposition of methane as the dominant component of natural gas. However, to control the decomposition process methane of the dispersed phase is extremely difficult. Therefore, its control on the residual methane or otherwise speaking, the degree of decomposition of methane. The process of decomposition of methane is determined by temperature and time. Temperature, at which the pyrolysis is carried out is determined by several factors. Chief among them during the thermal pyrolysis of methane its constant flow rate is the intensity of heat supply system, which finds concrete expression in the temperature, which is supported by the walls of the container in which there is expansion. Oxidative pyrolysis of a large influence on the process temperature has a coefficient of oxygen consumption.
Radiative properties of flame depends on the size and concentration of boxed soot particles suspended in the gas phase. All the gas can not simultaneously achieve a particular temperature, and some may microvolumes heated in different ways. Therefore, the decomposition products along with the intermediate products is present and a carbon black carbon.
Thus, the formation of carbon is through the formation of very large and possibly unstable molecules that have arisen as a result of the polymerization radicals and can further graphitized. Choice in practical terms the optimal coefficient of oxygen for the oxidative pyrolysis of methane determined not only by the desired degree of decomposition, but also the thermal characteristics of the process. In the expenditure part of the heat balance of the decisive role played by loss of heat carried away from the reaction zone gaseous products.
therefore advisable to conduct the process reformirovniya natural gas in the mode of oxidative pyrolysis with enriched air or even pure oxygen. Naturally, the temperature drop will be greater, the higher the degree of expansion, and the smaller, more heat has gas environment after the completion of the oxidation of methane. Therefore, the application of heated air will reduce the difference between the initial and final temperature of pyrolysis of methane atmospheric oxidation products of methane.
When heating the air up to 1100 ? C is an acceptable mode when = 0.15, as it gives a very significant amount of decomposition products and a large range of temperatures is practically self-supporting in thermal terms.
Use as a fuel partially reformed natural gas furnace shlakoplavilnoy capacity of 4 t/h of finished products, helps reduce fuel consumption by 30 - 40% and reduce the thermal processing of materials. Techno-economic calculations show that the return on fixed in the flue tract kiln reformer is less than one year.