Abstract on the master's work
Contents
1) Introduction.
2) Methods of intensifying the process of dewatering of fine coal.
3) Dispersion and stability of mineral suspensions.
4) A theoretical analysis of the mechanism of action of dehydrating agents, water repellents and flocculants.
4.1) Surface-active substances.
4.2) The mechanism of interaction of surfactants with carbon particles.
5) Trends and problems of research.
5.1) Theoretical studies.
5.2) Experimental studies.
6) References.
Introduction
In today's concentrators important part of the process is dehydration.
Dewatering operations of small dressing products can be divided into three groups:condensation and dehydration under the influence of gravitational forces, mechanical dewatering and thermal drying.
Methods for the intensification of these processes can be varied.
With increasing degree of mechanization of mining operations increased amount ofdetail, which is extracted and enriched. Of particular importance intensification ofdehydration has a small dressing products for mining and mining and chemical industries, due to the fact that the grinding is subjected to a deep almost all the raw materials that goes to the processing methods of magnetic separation and flotation, followed by dehydration. In practice, enrichment is used as a flotation for the enrichment of sludge, and for the timely removal of fine silt from the circulating water, which createsconditions for the closure of the water-sludge cycle. The number of small dressing products are subject to dehydration.
Significant prospects are physico-chemical methods of intensification of the existing equipment that will improve the efficiency of dehydration without significant capital investment. Experience of industrial chemicals, the introduction of intensifiers in themineral processing plants gave positive results. Thus, the use of reagent-intensifiers is one of the most important factors in addressing the improvement of water and sludgemanagement at the concentrators and the prevention of environmental pollution. As agents, intensifiers can be used inorganic electrolytes, low molecular weightsurfactants and synthetic petroleum, water-soluble polymers, and viscous organic liquids.
Methods of intensifying the process of fine coal dewatering
Methods of intensification of the processes of dehydration of small dressing products may be different. In this paper we consider only the physical-chemical methods of intensifying the processes of mechanical dewatering and filtration process mainly small dressing products. These techniques allow not only to increase the performance of filtration products, but also reduce the removal of solids from the filtrate and in some cases to reduce the moisture content of sediment. Reduction of flotation concentrate removal increases the yield of marketable products, and leads to improved technological performance enrichment processes in general by reducing the degree of zashlamlenosti recycled water.
In recent years, attracted the attention of specialists of the heat-physical methods of intensifying dehydration, which are in use during the dry sediment was deposited on the grid, the hot gas (products of burning fossil fuels, atmospheric air). Although the use of hot gas has the same goal as the heating of the suspension before filtration, it is not only more efficient but also economical. The advantage of this method is due? Primarily low-cost heat for heating a relatively small amount of water covering the surface of the inner cavities of sediment after the exclusion of these fluids. Most of the moisture due to its short duration of contact with the hot gas is heated slightly. However, this heating is sufficient to change the viscosity of water and the weakening of its energy to the surface of the material, resulting in a hydrodynamic flow of water under the pressure of the gas, which moves in the capillaries.
Sludge dewatering with superheated steam was carried out under industrial conditions.For this method is characterized by minimal loss of heat from exhaust air, high security and technological efficiency. In the superheated steam contains no oxygen, so theoxidation or combustion of coal is possible. Drying with superheated steam gives the effect of dehydration of the porous sediment.
|
Steam temperature, оС |
120 |
|
|
|
> 180 |
|
Humidity sediment, % |
25.3 |
21.0 |
18.6 |
18.5 |
17.0 |
Img 1 — Dependence of the moisture content of sediment on the temperature of superheated steam
Intensify the process of dewatering of fine coal filter apparatus can also change the structure of filter cake, or a decrease in the binding energy of the particle surface with water. Under the restructuring meant an increase in sediment porosity and a decrease in its specific surface area due to the aggregation of fine particles under the influence of coagulant (electrolyte) and flocculants (organic compounds and water-soluble polymers).Adding hydrophobic reagents according to the type of oils provides an increase in contact angle q, and the use of surfactants lowers the surface tension of water.
As a result, the height of capillary rise of water and the capillary pressure decreases, and therefore the number of small coal-held moisture decreases. The use of surface-active substances, together with a decrease in surface tension of water increases the wettability of the particles, and hence to increase the height of capillary rise of water in the sediment layer, filtered, and an increase in humidity. Thus, for better dewatering reagents must be used, which reduces the surface tension of water is reduced and the wettability of coal.
Coagulation of fine coal, which is preceded by its filtration, leads to the fact that the smallest particles, incorporating a relatively large floccules, increases the porosity of thesediment. Thanks to increasing its permeability and accelerates the formation of a layeron the filter. Surface-active substances under certain conditions contribute to the emergence of osmotic pressure, which increases the rate of fluid filtration and capillaryphenomena of osmosis, reduces the moisture content of sediment during drying. Based on the foregoing, we choose to intensify the process of fine coal dewatering of surface-active substances (chemicals, water repellents), and flocculants [2].
Dispersibility and stability of mineral suspensions
Slurry concentration and hydrometallurgical processes are polydisperse systems of particles with a size from one-hundredth of a micron to several millimeters. Depending on the size of the particles, which dominate, distinguish coarse-grained, fine-grained, silty shlamisti and pulp. In coarse-grained include pulp, which is dominated by particles larger than 0.5 — 0.3 mm. Fine-grained particles are smaller than the pulp with 0.5 — 0.3 mm.Referred to as sludge suspensions with particles of size less than 50 — 70 microns, and sometimes produce the most delicate part of the sludge — sludge with a particle size less than 10 — 5 microns. In the coal industry is often referred to the sludge particles less than 2 mm.
To include slurry pulp, which take precedence over other sludge particles quantitatively or qualitatively. At the same characteristics attributed to the clay slurry with appropriate quantitative or qualitative benefits of sludge. The boundary between the suspensions and colloids in the range size of 0.1 — 1.0 microns.
Thus, to include the actual suspensions dispersed systems with a predominance of particles larger than 5 microns. They are not colloidal dispersions, however, have much in common with the properties and much more practical value than the ash. When the acid leaching of gold ores and concentrates, non-ferrous, rare, radioactive and other metals are enriched suspension colloidal dispersions of silicon and aluminum acid. In alkaline slurry hydrates appear oxides of iron, aluminum and other metals. Stabilization of dispersed fractions of ore slurries by a variety of mechanical effects in the processes of treatment (intensive mixing, grinding), especially at elevated temperatures. Chemical peptization occurs when injected into the pulp chemicals that contribute to hydrophilization of the particles and increase their surface electric charges (water glass, the field of phosphate), reduced the concentration of the coagulating electrolyte by washing the precipitate with water, changing the other properties of the medium, which leads to an increase in the charge of colloidal particles and their dispersion .
The stability of dispersed systems is one of the central issues of colloid chemistry.Consideration of this issue of suspensions is associated with the intensification of the processes of condensation and filtration [1].
Theoretical analysis of the mechanism of action of dehydrating agents, water repellentsand flocculants
Surface-active substances
Surface-active substances are called chemical compounds, such that when dissolved or dispersed in a liquid selectively adsorbed at the interface, in turn, defines a set of physico-chemical or chemical properties that are of practical importance.
The main physico-chemical properties of surfactants, which are based on many processes, including those that run on concentrators, include: reduction of surface tension, surface activity, the critical micelle concentration, wetting and hydrophobization, emulsification, foaming, stabilization and others.
The properties of surfactant affects not only the number but also the order of connection of the individual atoms that make up molecules.
Properties, which are different surfactants are:
— Reduction of surface tension in a very dilute solution due to adsorption and orientation of the molecules at the interface;
— The formation of micelles in the destruction of the corresponding concentration of the solution
SAC by reducing the free energy of the system.
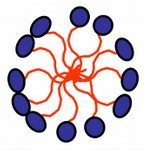
img 2 — micelles [5].
All synthetic surfactant is oil and water sensitive compounds, consisting of hydrophobicand hydrophilic parts.
Are part of the hydrophilic carboxyl-SOO, OSO-3 sulfate and sulfonate SO-3 groups, as well as a combination of hydrophilic residues with ether groups — CH2 — CH2 — O — CH 2 -CH 2 -, polyglycolic — (SN2SN2O) n, or groups that contain nitrogen.
The hydrophobic part consists mainly of paraffin chain, straight or branched, benzene ornaphthalene ring with alkyl radicals.
It can be argued that the use of surfactants is almost universal, because every production process in one way or another associated with the interaction of the surface.
Synthetic surfactants are used at present are divided into four classes:
— Anionic surfactants — compounds that dissociate in aqueous solution to form anions,causing the surface activity. Among them are the most important linear alkyl benzene sulfonates, sulfates, and sulfoefiry fatty acids;
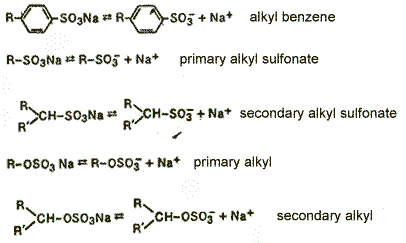
img 3 — anionic surfactants [5].
— Amphoteric (ampholytic) surfactants — compounds that are ionized in aqueous solutionsand lead, depending on conditions (mainly on the pH — medium), ie in acid solutionexhibit the properties of cationic surfactants in alkaline solution — anionic surfactants.Among the principal amphoteric surfactants should be noted alkylbetaines,alkilaminokarbonovye acid derivatives of alkyl imidazolines, alkilaminoalkansulfonaty;

img 4 — amphoteric surfactants [5].
— Non-ionic surfactants — compounds that dissolve in water, without ionizing. The solubility of nonionic surfactants in water is explained by the presence of functional groups. As a rule, they form the nitrates in aqueous solution as a result of hydrogen bonds betweenwater molecules and oxygen atoms of the molecule polietilenglikolovy surfactants. These include polyglycol esters of fatty alcohols and acids, polyglycol esters of fatty acid amides, alkyl polyglycol ethers alkylamides.

img 5 — Non-ionic surfactants [5].
— Cationic surfactants — compounds that dissociate in aqueous solution to form cations,determining the surface activity. Among the cationic surfactants are the most importantquaternary ammonium compounds, imidazaliny, fatty amines.
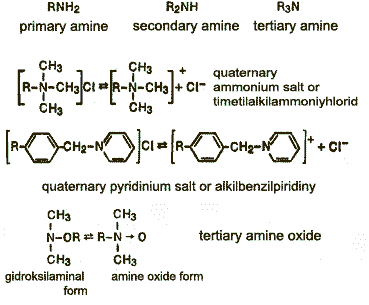
The mechanism of interaction of surfactants with carbon particles
Wetting of each surface flows better, the more liquid reduces the surface energy of the body is wetted, the lower this energy at the interface of two phases. Therefore, to reduce the wettability of coal with water, that is, for its water-repellency, it is necessary to increase the surface tension at the solid-liquid interface σ
From the equilibrium conditions should be:
where β — the surface tension of wetting, kg / m
The larger this value, the easier it is moistened with water and carbon the harder it is to dehydrate. With a decrease in the surface hydrophobicity of the attraction of wetting of coal increases. From the equation it is clear that for the intensification of dehydration is necessary to reduce the attraction to the surface of water at the water-air and increase the contact angle of coal. However, the magnitude of the contact angle is directly dependent not only on the surface tension at the coal-water, but also on the interaction of this magnitude with the forces of gravity on the surface of the partition of other phases.When dewatering of fine coal in the period of drying sludge on the filter surface is three-phase system, because after the separation of the main body of water in the sediment is always present air.
Among the impurities, which reduce the surface tension, foaming agent include (terpineol, phenol derivatives, aliphatic alcohols) and such surface-active substances such as alkyl sulfates, akrilsulfonat, turpentine, oil soap, fusel oil, synthol, Nekal, pasta AAS and P16, etc [4].
Directions and objectives of research
The review and analysis of the issue of small and dewatering of fine coal shows the following classes. First, the basic processes of mechanical dewatering, — drainage, centrifugation, filtration, screening, — who have the theoretical basis and are widely used in modern ore processing plants in terms of technology is almost exhausted. Second, the high specific physico-chemical methods of dewatering of fine coal and fine-grained, developed both abroad and domestic variants thereof are not communicated to a wide industrial use, apparently because of a number of deficiencies: bulkiness of special equipment, dorovizny used reagents their scarcity. In addition, the apparent lack of dewatering schemes using modern physico-chemical methods to ensure the weakening of bonds at the phase boundary coal-water
is applied at the final stages of dehumidified all the same traditional methods and mechanical dewatering devices are not able to realize the full effect Physical and chemical exposure. Thirdly, we must note the appearance and development of new long-term process of dewatering of granular material, in particular the fine coal — a method aeromechanical disruption of water injection in the film layer. However, deeper studies of this method to the raw coal is not carried out. The question of combining it with the physical and chemical methods remains an open question. Scientific rationale for hardware design has not been developed. The process is not prepared, nor in the scientific, theoretical terms, nor in the technology for use in modern concentrators. However, the trend toward deterioration of raw materials processed coal — increase the proportion of small and fine fractions in the original rock mass, an increase of ash content — requires the development of new high-enrichment processes and dewatering of coal. [2]
The aim of this work is the development and study of fine coal dewatering technology in the gas jet stream, development of recommendations, technological regimes and patterns of use of this process in the industry.
To achieve the objectives you must perform the following theoretical and experimental studies.
1. Theoretical studies
— To develop a structural model and the factorial process;
— To develop a theoretical interpretation of the effect in the dehumidified gas jet stream taking into account the preliminary physico-chemical effects on the interface coal-water
— To analyze the cohesive forces of water films with a surface coal mining;
— To analyze the factors intensifying dehumidified in a gas jet stream and to determine methods for effectively managing, efficient ways to influence the final result of dewatering of coal [7].
2. Experimental studies
— To determine the rational parameters of permanent dewatering of fine coal in a gas jet stream, in particular rational relationship air-to-solid
, pre-rational level of hydrophobic coal surface, the rational size of the source material;
— The method of the planned experiment to obtain a mathematical model of the dewatering process of fine-grained coal gas jet stream and perform its analysis;
— A comparative experimental evaluation of existing and emerging methods aeromechanical dewatering the coal feedstock, on the basis of which provide a promising area of use.
Three. In the development of industrial technology: to perform tests, industrial technology dewatering of fine coal in the gas jet stream, develop a version of the Industrial Technology dewatering of fine coal omaslivanie — ejection
for steam coals of Donetsk basin, to develop recommendations for the use of industrial technology aeromechanical dewatering of fine coal in a gas jet stream [7].
References
-
Майдуков Г.Л.
Технология фильтрования продуктов обогащения углей
, М.,Недра
, 1975, 144 с. -
Каминский В.С., Барбин М.Б., Долина Л.Ф.
Интенсификация процессов обезвоживания
, М.,Недра
, 1982, 224 с. -
Флокулянты —
Слаф Реагент
Флокулянты органические — синтетические полимеры (полиэлектролиты), используемые для механической очистки воды от взвешенных и коллоидных ... -
Статьи по КМЦ, NaКМЦ и обойному клею. — Давос-Трейдинг: КМЦ ...
8 авг 2011 — Механизм действия флокулянтов основан на следующих явлениях: адсорбции молекул флокулянта на поверхности коллоидных частиц ... -
Классификация ПАВ
ФИЗИКО-ХИМИЧЕСКИЕ СВОЙСТВА МОЮЩИХ ПОВЕРХНОСТНО-АКТИВНЫХ ВЕЩЕСТВ. 1.1. Классификация ПАВ. Все поверхностно-активные ... -
ПАВ
30 мар 2009 — ПАВ — органические соединения, молекулы которых имеют в строении полярную гидрофильную часть (функциональные группы —OH, ... -
Жужиков В.А.
Фильтрование.Теория и практика разделения суспензій
,М.
Химия
,1971,439 с.


