- The aim and the tasks of the work.
- The actuality of the work.
- Methods and algorithms to solve the tasks set.
- Conclusions
- List of sources
The aim and the tasks of the work.
The aim of the magister research work is projecting a specialized computer system (SCS) of building a three-dimension model of maxillofacial area based on the SCT data (spiral computer tomography) and defining the topographic anatomy parameters on the obtained model while preparing for the operation of dental implantation.
While doing this work the following tasks are to be solved:
- General analysis of the subject under research and setting the task;
- Reviewing and analyzing the existing systems and methods connected with the developing of the maxillofacial area images;
- Reviewing, analyzing and choosing the methods of image development;
- Analyzing and choosing the methods to build the three-dimension model;
- Designing the SCS structure, defining its subsystems and functional units;
- Choosing the medium of the software development according to the tasks set and methods chosen;
- Creating the SCS and approbating it in the real life conditions;
- Analyzing the results obtained at SCS work.
The actuality of the work.
Diagnostics, treatment and rehabilitation of the maxillofacial area injuries have always been one of the urgent tasks of the maxillofacial surgery. The maxillofacial area victims account for the 25% of the patients of the type clinics, there has been an increase in the number of the badly damaged bones of the skull which are combined in 10-12% of cases with other body and system injuries, the number of arising complications remains at quite a high level up to 15-25% [1].
In recent years computer technologies have actively entered different spheres of medicine. They are called not to replace the clinic doctor, but to give at his disposal as wide range of instruments, as possible to solve different clinical tasks.
A three-dimension model of a certain area of a maxillofacial based on the results of the spiral tomography can become such a technology in the sphere of maxillofacial surgery, dental surgery in particular [2].Having such a model at his disposal a clinic doctor can more accurately estimate the anatomic peculiarities of a particular patient, localizations, bounds and extenrt of the pathological process, plan the measures of the operation and value the results of the executed treatment.
That is why the actual task is to develop a specialized computer system (SCS) of defining the topographic anatomy parameters of a bone on the spiral computer tomography data (SCT) which will determine on the obtained data the most suitable implant from the data base and execute its positioning in the 3D model of a maxillofacial area. Using such SCS will enable to represent as fully as possible the bounds, the size and the volume of the defect(deformation), plan the operation process and reduce the risk of post operation complications appearance.
Methods and algorithms to solve the tasks set.
The result of conducting the SCT operation is an array of layer slices in DICOM format. A DICOM format file represents a set of tags and pixel values which enable to execute the visualization of the image [3].
To determine the required parameters (bone’s width and height, as well as bone’s tissue density) it is necessary to realize the possibility of building the cross-section on all the images in the place where the implant is supposed to be installed. The place of installation is determined by the doctor manually through drawing a cross-section line on the graph image. As a result of building the cross-section we obtain the image, where the required area of a jaw is shown without any form or size distortion.
Building the cross-section graph comes to the task of creating the image on the interim lines. Each line corresponds with the pixels of a particular CT graph, and the meanings of lines between the graphs (non-informative lines) are filled with the meanings corresponding with the black color.
The number of non-informative lines to one slice can be calculated on the formula (1), proceeding from the information (tags), which is contained in every DICOM file.
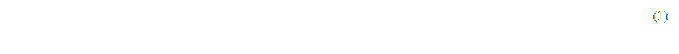
where K is number of non-informative lines per slice;
D – physical distance between the slices (tag (0018, 0088) – Spasing Between Slices);
T – slice's thickness (tag (0018,0050) – Slice Thickness).
Accordingly, the height of the obtained cross-section image is determined on the formula (2).
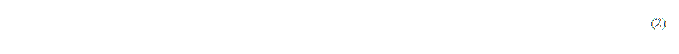
where H is the height of the image in pixel;
z is the number of graphs on which the cross-section is built.
The width of the crass-section image represents the length of the drawn line, which can be seen as a hypotenuse to a right-angled triangle. Then the number of pixels in a line is determined as the maximum of the legs of a triangle meaning (3).
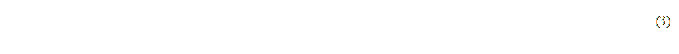
where W is the width of the image in pixels;
dx, dy – is the number of pixels of the corresponding leg of the right-angled triangle.
The density of the bone tissue is determined in the area, which corresponds with the place where the implant is to be installed. This area can be determined in two ways: through the automatic marking and if the results of the first methods are not satisfactory, through manual marking of the particular area. As a method for automatic marking we use the methods of spreading the areas from the seeds [4]. The seeds mark the objects which are to be marked. The areas grow gradually, comparing all the non-occupied neighboring pixels with the current region.
As a measure of similarity we use the difference between the pixel brightness and the average brightness of the region. The pixel with the smallest such a difference is added into the corresponding region. The process continues until all the pixels are added into one of the regions.
Then there is a review of all the pixels that belong to informative lines, inside the obtained area and the sum of their meanings is found. The obtained sum is divided by the number of the summary pixels and thus the arithmetical mean of pixel brightness in the marked area is found.
The density of the bone tissue is expressed in the Hounsfield scale units [5], to transfer into which the obtained average pixel brightness meaning must be calculated on the formula (4).

where HU is the meaning in Hounsfield units;
PV is the average meaning of the pixel brightness in the area;
Slope – (tag (0028, 1053) – Rescale Slope);
Intercept - (тег (0028, 1052) – Rescale Intercept).
To determine the height and the width of the bone it is necessary to obtain the meaning of the line drawn by a user in physical units of a length. Accordingly it is necessary to calculate the physical size of a pixel (width and height).
The height and the width of an image cross-section pixel in physical units of a length can be calculated as the relation of the real height/width of the built slice array of SCT graph to the number of pixels, which are used to show the height/width of the graphs cross-section array (5).

where RSizePix is the real size of a cross-section pixel (height and width);
RLength is the real size of the cross-section;
PixelNmb is the size of the cross-section in pixels.
Then the length of the line, which defines the width or the height of a bone is calculated on the formula (6).
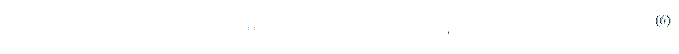
where Length is the real length of a drown line;
RSizeХ, RSizeY is the physical width and height of a slice image pixel;
dx, dy is the number of pixels of the corresponding leg of the right-angled triangle.
The area of building a 3D model in space is represented by a parallelepiped situated inside the other one, composed by a full array of a patient’s current check up graphs. Such a parallelepiped is set by minimum two rectangles, each per projection, which are set with the help of parameters: Height, Width and Length. Each of these parameters can be calculated on the following principles (7).
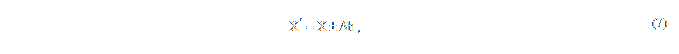
where X’ is the size of a corresponding rib of a parallelepiped;
Х is the size of the implant's corresponding parametr;
h is the distance increasing the size of the area in two directions.
These rectangles are connected to each other by the size of one of the sides, which is a joint edge of the parallelepiped, as well as by the coordinates. Accordingly, changing the Y coordinate on the horizontal projection is transformed in changing the X coordinate on the profile projection.
As the algorithm to building a 3D model we use the altitude suggested in this work [6]. To realize the task set the algorithm has been modified. Thus, instead of a 3D model with fixed parameters of the initial data ( the width and the length of the image, the number of images in the graph array) a model with settable parameters is created. It means that the width and the length of the image area used in creating a 3D model, as well as the meaning of such images are set according to the meaning of the implantation parameters defined previously.
Conclusions
This article formulates the main aims and tasks which are solved in the process of designing the SCS of building a 3D model of the maxillofacial area and defining the topographic anatomy parameters of implantation. It considers and analyzes the existing world and national achievements in this sphere, points out their advantages and disadvantages, defines the requirements to be met by the SCS developed and the practical results planned.
The obtained as a result of the SCS work set of topographic anatomy parameters (density, length and width of the bone in the place of the injury, it used make as accurate as possible choice of the dental implant’s type and size the stage of preparing for surgical operation.
The result of building a 3D computer model of the problem area of a maxillofacial area together with the size and the localization of the implant visualizes the results of processing the patient data, which are given to the doctor's consideration for father usage while doing the operation.
When writing the abstract of the master's work is not yet complete. Final completion: December, 2013. The full text of the work and materials on the topic can be obtained from the author or his scientific adviser after that date.
List of sources
- Д.К. Калиновский, И.Н.Матрос-Таранец. Современные подходы в диагностике, лечении и реабилитации травм челюстно-лицевой области с использованием компьютерных технологий и телемедицины. Том 7, №1, 2009.
- Д.К. Калиновский, А.Н. Чуйко. Возможности использования современных компьютерных технологий CT/CAD/CAM в челюстно-лицевой хирургии. Український журнал телемедицини та медичної телематики. Том 9, №1, 2011.
- DICOM Standard [электронный ресурс]. - Режим доступа: http://medical.nema.org/standard.html
- Gonzalez R. C., Woods R. E. Digital Image Processing. Prentice Hall 2002, pp. 813.
- Converting CT Data to Hounsfield Units [электронный ресурс]. - Режим доступа: http://www.idlcoyote.com/fileio_tips/hounsfield.html
- В.Г. Адамов, К.В. Меркулова, О.Л. Толстих. Моделювання тривимірних об'єктів для збільшення ефективності проведення остеосинтезу // Вестник Херсонского государственного технического университета. – Херсон: ХГТУ, 2012. – № 1(44). – С. 323–331.
- Hounsfield scale [электронный ресурс]. - Режим доступа: http://en.wikipedia.org/wiki/Hounsfield_scale
