Abstract
Сontent
- Introduction
- 1. Review of known technologies degassing of molten metals in the electrostatic field
- 2. Description of the technology degassing molten aluminum from the hydrogen in the electrostatic field
- Conclusion
- References
Introduction
This master thesis is devoted to the study of the processes of mass and heat transfer during the degassing of molten aluminum from the hydrogen in an electric field.
Intensification of metallurgical technologies, leading to an increase in equipment productivity, in particular, secondary treatment melts - the actual scientific field.
Intensity diffusion remove hydrogen from the liquid aluminum in absolute value:
|q|=D(T)/s•(Cm-Cn),
where D (T) - the diffusion coefficient of the gas in the melt increases with the (T) of the temperature of the molten metal; Cm, Cn - the concentration of this gas in the melt at the interface and the "vacuum - metal»; s - thickness of the diffusion boundary layer at this boundary.
To increase | q | at a fixed temperature of the metal should: a) reduce s by melt mixing (eg, argon blowing through the porous plug mounted in the bottom of the bucket with a metal), and b) reduce Cn by using vacuum. If the vacuum - the camera put the source of the electrostatic field (E> Ekr, Ekr - critical value of E), it rips protons (hydrogen ions are removed) from the surface in substantially reducing Cn.
1. Review of known technologies degassing of molten metals in the electrostatic field
For static melt technology experimentally investigated in [1]. Analytical review of the literature, and scientific premises on the effect of the electrostatic field on the intensification technology degassing ladle of liquid metal rolling (for molten steel) are given in [2,3].
Very effectively influence the electrostatic field on the melt during degassing [1,4,5]. For desorption of ions in the vacuum space, they need to report the activation energy, more energy to the surface of the metal. This energy is imparted external electrostatic field. In this case, the union of two atoms in a molecule facilitates desorption of the cavity vacuum vessel.
Mechanical stresses in the adsorbed layer under the influence of the external field strength Eo are of the order of Eo 2 / 8P [1]. Therefore, no matter how high was the strength of the particles to the surface of the metal, increasing Eo can always create tearing voltages exceeding this strength. As a result, it becomes possible to remove adsorbed particles from the surface of the melt.
The results show that the elements and compounds in the melt are partially in the form of ions. This gives a basis for control by an electric field behavior of these particles in the process of refining the metal.
Theoretical basis refining still melts in the electric field are given in [1]. According to these studies, varying the voltage and polarity of the electric field can influence the direction of the kinetics and transport processes at the boundaries of the "gas - metal».
The additional involvement as factors influencing the temperature and pressure of the gas above the melt significantly enhances control the refining process.
2. Description of the technology degassing molten aluminum from the hydrogen in the electrostatic field
Electric field affects the processes of decontamination of metal, mainly due to changes in the concentration of exhaust gas (eg hydrogen) on the surface of the melt, which is included in the calculation formulas as diffusion and adsorption-kinetic component of the mass flow of the gas.
Therefore, the development of technologies relevant to the metal complex influence of various factors.
Investigation of mass and heat transfer was carried out on the basis of computer modules developed by the finite-difference method of alternating directions with a uniform grid in cylindrical coordinates.
In computer modeling of mass and heat transfer in a liquid bath in effect at the interface of "vacuum-metal" high voltage electrostatic field (E> Ekr) that the external field is a rip off of the atoms are ionized hydrogen (protons) with the considered the interface.
Hydrogen concentration on the surface:
Cn = K H • sqrt (P H2 ) [(1-i) i • K E ],
where K H - Sieverts constant for hydrogen; P H2 - its partial pressure in the cavity vacuum vessel; i - ion fraction; K E (r) - the distribution of protons on the surface of the "vacuum-metal».
When E> Ekr function K E (r) = 0, so the above relation takes the form:
Cn=KH•sqrt(PH2) (1-i),
The program is based on the following values laid PC i = 0,1; 0,5; 1,0. As the results of computer simulation, a significant intensification of the diffusion process, even when i = 1,0 happens (especially when i = 0,1 and 0,5).
Much greater interest to the industry is the region of moderate values of the electrostatic field.
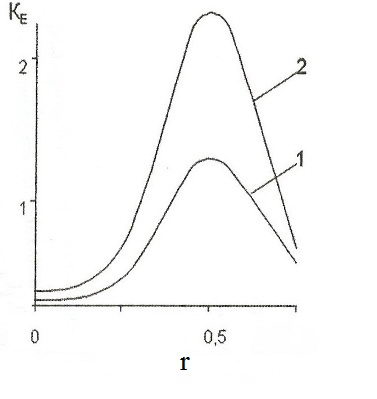
Dimensionless function K E (r), which determines the degree of increase in the concentration of hydrogen atoms Cn (i = 1) at the interface of "vacuum-metal" with exposure to this area of the electrostatic field in comparison with the value of Cn 0 (for E = 0) is shown in Fig. 1.
Figure 1 - Distribution of the concentration of hydrogen ions at the interface: 1-E = 1 • 10 5 V/m; 2 - E = 2 • 10 5 V/m, i = 1.
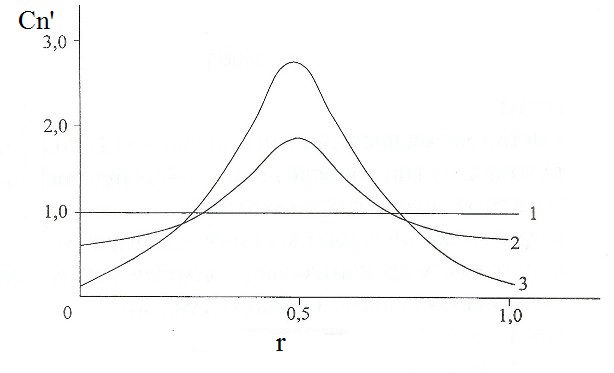
Given K E (r) by the formula of hydrogen concentration on the surface of a "vacuum-metal" build distribution schedule considered dimensionless concentration Cn'= Cn/Cn 0 in the coordinate r surface (Fig. 2).
Figure 2 - Distribution of the dimensionless concentration Cn'of hydrogen atoms at the interface "metal-vacuum": 1-i = 0, 2-i = 0,5; 3-i = 1,0; E = 2,0 • 10 5 V/m
As this graph, in the projection of the source of the electrostatic field, that is cylindrical electrode, the molten aluminum mirror has a maximum Cn', the value of which increases with the degree of ionization of hydrogen atoms i.
If degassing of molten aluminum hydrogen limited kinetic act molizatsii (low concentration of hydrogen atoms in a liquid bath or a high concentration of surface-active elements), is a chemical element with our technology significantly increases, intensifying technology. This follows from the known dependence of the increase in the second degree of the reaction rate molizatsii atoms or ions remove hydrogen at the interface of "vacuum-metal" on their concentration on the surface. Thus, the increase in the concentration of 3-fold (see Fig. 2) leads to an increase in the rate of chemical reactions in 9 times.
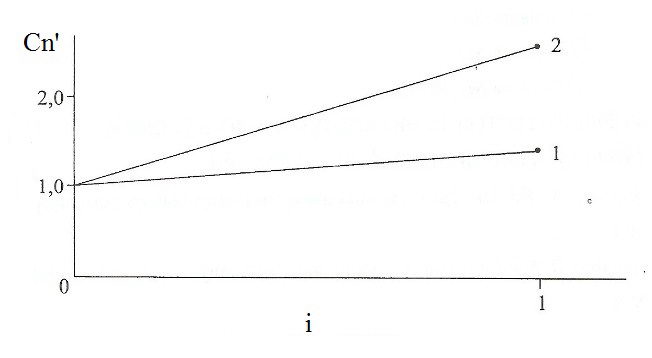
Fig. 3 shows the maximum Cn' of the electrostatic field. Its increase leads to an increase of this magnitude and degree of amplification of the kinetic level of the technology.
Figure 3 - The relationship between the maximum dimensionless concentration Cn' of hydrogen atoms at the interface "metal - vacuum" of the degree of ionization: 1-E = 1,0 • 10 5 V/m; 2 - E = 2,0 • 10 5 V/m
Conclusion
An analysis of the literature, the region of moderate values of the electrostatic field in the study of the technology for the first time. In addition, the question of degree of ionization of hydrogen atoms in the molten aluminum is also not changed. As the results of computer simulation, if degassing blocked kinetic weak link, the connection electric field can resume the technology up to the concentration of hydrogen in molten aluminum to very low values. It is important for the production of aluminum of high purity, ie the highest quality.
In writing this essay master's work is not yet completed. Final completion in January 2014.
References
- Кайбичев А. В., Лепинский Б. М. Рафинирование жидких металлов и сплавов в электрическом поле. – М.: Наука. – 1983. – 120 с.
- Захаров Н. И., Троцан А. И., Овдиенко А. А. Об использовании электростатического поля в технологии внепечной дегазации стали // Процессы литья. – 2009. - №1. – С. 8-11.
- Захаров Н. И. Интенсификация массообменных процессов внепечной дегазации стали // процессы литья. – 2010. - №4. – С. 8-12.
- Кайбичев А. В., Алешина С. Н. Эмиссия металлических расплавов в электростатическом поле // Строение и свойства металлических и шлаковых расплавов. – Екатеринбург. – 1998. – С. 22-23.
- Дюдкин Д. А., Захаров Н. И. К вопросу энергосбережения при дегазации металла // Металл и литье Украины. – 1996. - №3. – С. 17-18.
- Семыкин С.И.,Поляков В.Ф. Применение электрической энергии малой мощности при выплавке металла//Сборник трудов 1-го конкурса сталеплавильщиков.-Липецк.-1992.-С.105-107.
- Семыкин С.И., Поляков В.Ф. Исследование металлургического процесса при воздействии электрической энергии//Известия вузов: Цветная металлургия.-1992.-№10.-С.6-8.
- Семыкин С.И., Поляков В.Ф., Учитель Л.М. Исследование влияния электрической энергии малой мощности на эффективность внепечной обработки металла//Металлы и литье Украины.-1995.-№7.-С.17-22
- Явойский В. И., Баталин Г. И. Удаление водорода из металлов в электрическом поле // Сталь. – 1954. - №6. – С. 5-6.
- Алюминиевые сплавы. Справочник. – М.: Металлургия. – 1974. – 432 с.