ABSTRACT OF THE MASTER'S TOPIC
Content
- Introduction
- 1. General information about the brasses and their properties
- 2. Deformed brass
- 3. Thermomechanical processing of brass
- Findings
- References
Introduction
In ancient times people first used metals that are encountered in pure form - copper and gold. Materials defined historical period: stone - copper, bronze, iron veka.Istoriki isolated stone-copper age when weapons and tools were made of copper and is used flint tools. Pervyemelkiemednyeizdeliya (arrowheads and spears) forged and finding nuggets. People found that in cold forging copper not only takes the desired shape, but becomes harder and prochnee.Pozzhe found that reinforced Cold forging metal can again make soft when heated it in the fire. It took a long time and people have learned plavitmed iotlivatee in shape, and just have to solder copper silver wire. Odnakoznaniya of metals and the ability to take off the handle is not in the public domain. Terms posvyaschennyhbyluzok
Kakizvestno, tsvetnyemetally alloys all shireprimenyayut in various fields of national hozyaystva.Mnohimi valuable qualities have non-ferrous metals such as copper, nickel, zinc, copper and so on silver is the best conductor of current, which accounts for its wide application in electrical engineering. Copper is also the basis of many important industrial alloys brass, bronze, etc. Due to the high mechanical and technological properties of the alloys of copper and zinc (brass) are the most common alloys of copper
1. General information about the brasses and their properties
An alloy of copper and zinc are called brass. By type of brass resembles gold. Brass is used in time of Homer (V III century BC) in ancient times float copper from zinc ore. And in Rome during the reign of Augustus (63h. BC - 14 AD) minted brass coins. Europe learned of zinc only in the 18th century. The Chinese zinc was known before/
Brass - an alloy of copper, in which the main element is lehyruyuschym zinc. They mark the letter L and the numbers that characterize the average content of alloying elements. Yes, brass L80 containing 80% Cu and 20% Zn. If brass lehirovanapomimotsinka other elements placed after the letter L symbol of these elements: C - lead, OH - tin, iron-F, A - Aluminum, K - Silicon, Martyr - manganese, N - Nickel. The numbers after the letters indicate the average content of each alloying element in brass, in addition to zinc. The content of zinc is determined by the difference to 100%. Yes, brass LAN-59-3-2 contained 59% Cu, 3% Al, 2% Ni and 36% Zn. In brass foundry mark does not indicate the content of copper and zinc, and the content of alloying elements indicate not end brands and poslebukvy indicating the item. Copper alloys have two features that must be considered when designing technology heat treatment: vysokuyuteploprovodnost and aktivnoevzaimodeystvie of gases when heated. Because zavysokoy heat in uprochnyayuschey copper alloy heat treatment there is no problem hardenability. When used in practice, dimensions and semi-products are prohartovuyutsya through. Brass actively interact with oxygen and water vapor at elevated temperatures, because heat treatment products and semi-finished products often primenyayutzaschitnye atmosphere: wet iosushennyyekzohaz, ekzomonohaz, monohaz, dissotsiirovannyyammiak..
Brass are widely used due to a combination of high mechanical and technological properties. Latuniotnosyatsya the system Cu-Zn diagram is shown in Figure ris1.1.V of Cu-Zn formed five peritectic transformation, resulting in the formation of five phases: alpha, beta, gamma, sigma, theta.
The negative feature brass is their tendency to spontaneous corrosion cracking that occurs vovlazhnoy atmosphere while maintaining the alloy after deformation residual stresses. Development cracking atmosphere cposobstvuet presence of traces of ammonia, ammonium salts, sulfur gases. This is known as a seasonal disease, as it often occurs in spring and autumn when air humidity is due povyshena.Rastreskivanie best brass corrosion along the grain boundaries in the area of non-uniform stress distribution. This phenomenon increases with increasing zinc content and growing rapidly especially in the maintenance of its more than 30%. To eliminate susceptibility to cracking rather otzhech deformed semifinished at temperatures below the recrystallization temperature. In this annealing effectively removed residual stress and retained high strength due to nahartovka.
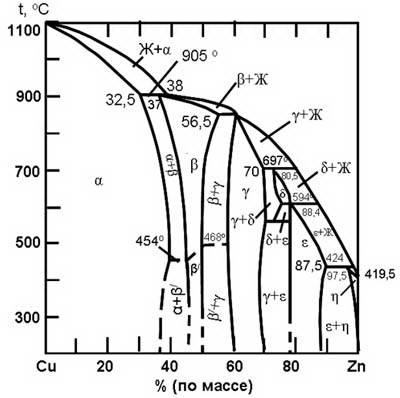
Figure 1.1 – Diagram of the Cu - Zn
Brass divided into binary (simple) and compound. Dvoynyelatuni is an alloy of copper and zinc. Many component brass kromemedi and zinc, containing one or more other alloying metals (aluminum, nickel, iron, manganese, tin, silicon and lead) Brass heavy non-ferrous alloys are the most common in modern engineering. They handled the pressure well and have a very high mechanical properties.
2. Deformed brass
Naibolshee rasprostranenie got bohatyemedyu alpha - brass containing up to 4% A1 (LA85-0, 5, LA77-2), which are due to single-phase structure is well handled pressure.
For nikelevoylatuni LN65-5 характернывысокиетехнологическиесвойства, onaotlichnoobrabatyvaetsya hot and холодномсостоянии.Марганцевая латунь LZhMts59-1-1 obladaetvysokoyprochnostyu and due to alloying iron. Tin Brass characterized by high corrosion resistance in seawater, so they are called marine brass.
Aluminum Brass LAMsh77 alpha-2-0, 05 by micro alloying arsenic well resists hanichnym and technological properties of the alloy of copper and zinc (brass) are the most common of copper with afloat. obestsinkovaniyu in seawater.
Lead brass handled well by cutting. This is the best material for brass parts vytochuvaty-processing machines. Unlike alpha - brass lead in the (alpha + beta)-Copper is not harmful impurities because rezultateprevrascheniya beta-alpha during cooling it is not located at the grain boundaries and inside the crystal alpha - phase formed on the germ vklyucheniyahsvintsakak . Lead makes chips brittle, making it easier machinability by cutting. At the same time lead increases friction properties.
Brass LANKMts75-2-2 ,5-0 ,5-0, 5 - the only variance solidified based alloy system Cu-Zn.
Provide dispersion strengthening connection osnovekremniya, nickel and manganese, which have variable solubility in copper. In the hardened state, this brass has high ductility and after aging shall vysokuyuprochnost. More high strength and elastic properties are achieved during aging of the brass after deformation in the hardened condition.
Properties brass strength can improve nahartovka. Tensile strength brass with naklepeuvelichivaetsya 250 ... 300 MPa. However, most libel brass, both simple and special, causes the development of their spontaneous cracking.
3. Thermomechanical processing of brass
Plastic deformation alters the distribution and increases the density of the crystalline structure imperfections - dislocations, vacancies, defects package was - and vysokouhlovyh borders. Since lattice defects strongly influence the structure formation in the alloy phase transformations, the plastic deformation phase transformations before or during their development can be used to create the optimal structure of heat-treated alloy.
Thermomechanical treatment (TMT) - a heat treatment that involves plastic deformation, which is due to high density of defects affects the structure formation during phase transformations that occur during thermal exposure.
So to deformation heat treatment can not be attributed any combination of operations deformation, heating and cooling. For example, if plastic deformation is performed after all heat treatment operation, we are not dealing with TMO as with conventional heat treatment followed by treatment with pressure. This plastic deformation such as cold rolling after aging can create libel increase prochnosnye properties, but it does not affect the formation struktkry under phase transformations because these transformations Strait to deformation.
If the plastic deformation was conducted to treatment, but has not made a defining impact on the formation of the final structure of the alloy during phase transformations, it is a combination of plastic deformation and subsequent heat treatment is not possible refers to the TMO. For example cold rolling, followed by heating for quenching, in which the recrystallization proholosta not yavlyaetsyasostavnymi parts TMO as recrystallization structure is characterized by low density crystalline structure imperfections.
The processes of plastic deformation and heat treatment at TMO can be combined in a single technological operation, but can be conducted at different times, for example, with a break of a few days. It is only important that at this phase transformations took place in high-density lattice defects created by plastic deformation.
Currently, the industry used and tested various schemes TMO, including hot and (or) cold plastic deformation, which makes a decisive influence on the structure of the alloy during aging and other transformations.
With regard to the variance tverdeyuschym alloys TMO in the industry engaged in the following tehnolohych. schemes: a) heated to temperatures ri hardening, deformation, immediate quenching, aging (VTMO), b) hardening, deformation, aging (NTMO). In implementing the second circuit may be difficulties associated with high resistance to deformation of the solid solution at room temperature reform. This scheme has several advantages: there is aging with the formation of highly dispersed phases even when cold (or warm) strain, creating a more uniform distribution of precipitates hardening phases formed on dislocations throughout the volume of grains. Second TMO scheme has been successfully used to increase the strength of aging copper alloys.
Stage plastic deformation was found primarily on single crystals. In 1930 D. Sachs, and D. Virts found to strengthen the linear (q = const) on single crystals of Cu, Ag and Au. In fact, this was the first report stage II to dependencies of stress-strain. G. Taylor and C. Elam in 1963 observed parabolic dependence of t - e on metal crystals. Stage of parabolic hardening stage name was subsequently III. Along with foreign authors (and in some ways, and ahead of them) conducted their research Leningrad scientist A. Stepanov, who discovered the three stages of building on ionic crystals. Unfortunately, the importance of this work was recognized by the community of scientists much later. Gradually improved technology for single crystals, and increased purity metal crystals. At that time, EM Andrade and co-workers discovered the stage with zero rate of strain hardening (stage I). This stage follows immediately after the yield strength, called stage lung skolzheniya.Izvestno that plastic deformation of metals is accompanied by accumulation of linear defects - dislocations. A detailed understanding of the dislocations and methods of observation can also be found in the article by specifically devoted to this issue. Quality communication pattern of distribution of dislocations in the sample observed by using a transmission electron microscope, with the stages of deformation have been recognized by Alexander Hovy and described by him in a series of publications in the years 1961-1962. Over the next three decades made a series of works in this direction foreign and domestic researchers. An important outcome of this research was to create a classification of the observed dislocation structures and sequencing of their evolution during deformation [3, 6]. It was found that with increasing deformation of metallic materials increases not only the number of dislocations, but the type of their spatial distribution. These specific types of dislocation distributions substrukturami.Odnim called dislocation of the important parameters of the dislocation structure is the dislocation density r, which is stored in the material during its deformation. The density of dislocations is the total length of dislocation lines per unit volume of the material [9]. This value is called a scalar density of dislocations, as in this case, the count density of dislocations is not the sign of dislocations. Meanwhile, dislocations can be of different sign (+ or -) [7, 8]. Sign dislocations are sufficiently conditioned, ie, some deployment should be considered positive, and some - otritsatelnymi.Znak disposition determined on the basis of its Burgers vector.
It should also be borne in mind that the TMO variance hardens solid solution in parallel with the diffusion redistribution of atoms during aging pre naklepanoho alloys redistribution of defects in the crystal structure. These processes are interrelated and mutually determine each other, resulting in their optimum combination in a significant strengthening. It should be noted that this duty plastic deformation and heat for copper alloys was once the only effective means of changing their mechanical properties and, above privacy in increasing resistance to plastic deformation. Found that the intensity of the strengthening of hardened alloy after deformation and reheating depends on: 1) the conditions of melting (purity) alloy 2) temperature and strain directions, and 3) the grain size, and 4) the time from the end of deformation before reheating 5) strain rate (strengthening is greater, the higher the rate of deformation).
Plastic deformation of all investigated alloys (after training) causes the disintegration of the solid solution, which is to reduce the electrical resistance and changes persecuted alloys. Hardness and elastic limit in this case is significantly increased. In brass with no defects detected deformation stacking.
Thus deformatsyonnaya heat treatment is the best way to improve the properties of brass.
Findings
In view of the above it can be said that the use of thermal deformation brass is one of the few effective ways to ensure a high mechanical properties of the alloy. However, the solution to this problem of the practical realization became possible recently due to new developments in the field of research modes termomehanichesoy processing..
- It was established that the most exposed alloys to deformation processing are double brass.
- recrystallization annealing is one of the most common maintenance for brass that allows you to continue to hold the hot plastic deformation
Found that plastic deformation before or phase transformations during their development can be used to create the optimal structure of heat-treated alloy
References
- 1. Konev NA Physics of the strength of metals and alloys / / Soros Educational Journal. 1997. Number 7. S. 95-102.
- 2. A.N. Orlov Introduction to defects. M.: High. wk., 1983. 145 with
- 3. Maltsev MV Металлография промышленных цветных металлов and alloys. / Maltsev M.V.-M.: Metallurgy, 1970.-364s
- 4. Konev NA Classification, evolution and self-organization of dislocation structures in metals and alloys / / Soros Educational Journal. 1996. Number 6. S. 97-107
- 5. Sergeyev VM Continuous casting-pressing of non-ferrous alloys / / VM Sergeev, Y. Peas. - Moscow, Metallurgy, 1990.-85c
- 6. Zholobov VV Non-ferrous metals and alloys pressure. / Zholobov VV -. M: Metallurgy, 1995.-486s
- 7. Bernstein ML Thermomechanical processing of metals and alloys. / Bernstein ML-M: Metallurgy, 1968.-486s.
- 8. Novikov IM The theory of heat treatment of metals. / IM Novikov M: Metallurgy, 1978.-392 with.
- 9. Smiryagin AP Nonferrous metals and alloys. / Smiryagin AP-M: GNTI, 1956.-560c.

