Abstract
Content
- Introduction
- 1.Overview
- 2.Description of diagnostic systems of hydrodynamic conditions of the reactors CNM
- Conclusion
- List of sources
Introduction
The problem of interaction between nature and society became crucial at this stage. Today it has become apparent that the task of preserving the environment and economic development are interrelated:destroying and depleting the natural environment can be no sustainable economic development.The idea of ??environmentally friendly technologies and industries, which arose as a result of awarenessmankind limited natural resources for economic growth,as well as the imminent danger of irreversible adverse changes in the environment,has found wide acceptance in the world. Many researchers agree that the development ofNanotechnology will largely solve the mentioned problemsdue to higher levels of resource in many industries.
There is a lot of information about the synthesis of carbon nanomaterials (CNM)in particular, carbon nanotubes (CNT), using various methods (electric arc deposition,CVD-synthesis on the catalytic pyrolysis catalyst with a substrate surface, etc.).However, the most optimal operating conditions of each reactor toconduct a study of influence of various process parameters on the intensity of theflowing hydrocarbon base decomposition reactions so, for example, many studies have shownthat the pressure in the reactor can substantially definethe rate of the synthesis, the diameter of the resulting nanotubes and their quality performance.
Master's thesis is devoted to the development of an expert system for the analysis of hydrodynamic parameters and diagnostic systems to createpossibilities of industrial synthesis of carbon nanotubes by catalytic pyrolysis,not having a hardware stationary flow meters and gas analyzer. In contrast to the methods mentioned above, has several advantages, such as aravnitelno low energy process, the use of cheap and available carbonaceous materials; "soft" technological parameters of synthesis, ease of construction andmanufacturability of the equipment used, the lack of need for expensive purification from impurities.
1. Overview
Carbon nanotubes - is elongated cylindrical structure with a diameter 1 .. 10 nanometersup to a few centimeters, consisting of one orrolled in a tube of hexagonal graphite planes and usually endinghemispherical head which can be seen as half of the fullerene molecule.Carbon nanotubes were discovered in 1991 by Japanese researcher Iijima.First nanotube was obtained by evaporation of graphite in an electric arc.Measurements made using an electron microscope revealed that the diameter of such threads ismore than several nanometers and a length of one to several microns.
By cutting along the longitudinal axis of the nanotube, it was found that it consists ofone or more layers, each of which is hexagonal mesh graphitewhich is based on a hexagon arranged at the vertices of the angles carbon atoms.In all cases the distance between the layers is 0.34 nm, i.e. the same as in between the layerscrystalline graphite. The upper ends of the tubes are closed with hemispherical caps, each layerwhich is made up of hexagons and pentagons, resembling the structure of the fullerene molecule halves.
Нанотрубки являются членами семьи фуллеренов, которая также включает в себя сферические фуллерены. Диаметр нанотрубки на порядок нескольких нанометров (примерно 1 /50, 000 ширины человеческого волоса), в то время как они могут быть до 18 сантиметров в длину (по состоянию на 2010). Прикладная квантовая химия, в частности, орбитальная гибридизация лучше всего описывает тип химической связи в нанотрубках. Химические связи нанотрубок полностью состоят из SP2 связи, подобной графиту. Эти связи сильнее, чем SP3, они и обеспечивают нанотрубки их уникальной силой. Кроме того, нанотрубки естественно объединяются "канаты" удерживающиеся вместе силами Ван-дер-Ваальса.
2. Description of diagnostic systems of hydrodynamic conditions of the reactors CNM
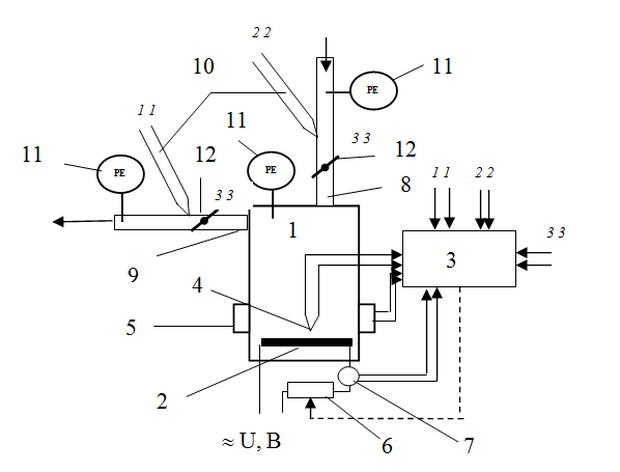
Figure 1 shows one of the traditional schemes of a batch reactor for the synthesis ofCNM on substrates with a catalyst: a cylindrical reactor (1) has connections for the supply anddischarge gases, at the bottom of the reaction zone is an electric heater (2)allowing maintain a predetermined temperature is monitored by a special thermocouple (4). According to this scheme applied to the problem of the study items such as instrumentation and control thermocouple pressure sensors. At the outlet and inlet pipes are pressure regulators - butterfly valves. The rotation angles of the throttle valve is converted into an electric signal by Resolver sensors. All signals from the sensors are transmitted to the controller.
Fig. 1. Block diagram of the diagnostic system of hydrodynamic conditions of the reactor CNM
(1 - reactor 2 - electric heater; 3 - Controller 4 - Temperature of the reaction zone;5 - dielectric sensors reaction space 6 - rheostatto control the heat output released by the heater of the reactor;7 - a device for measuring the power consumption of the heater, 8 - the intake of hydrocarbon;9 - pipe removal of gaseous products, 10 - Thermocouple, 11 - pressure sensors, 12-throttle valves;1-1, 2-2, 3-3 - signals from the respective sensors).