Аннотация
The results of thermogravimetric and XRD studies of thermal decomposition of oxalate precursor of morphotropic PZT solid solution Pb(Zr0.52Ti0.48)O3 are presented. A two-stage schedule of heat treatment was proposed: dwelling the precursor at 380 °C and quick heating and dwelling at 800 °C. Using this regime a homogeneous PZT solid solution has been synthesized.
Introduction
Due to their exceptional electrophysical properties, lead zirconate–titanate (PZT) solid solutions [1] remain for decades the most widely used piezoelectric ceramic materials. PZT exhibits noncentrosymmetric perovskite structure and many its properties reach maximum at the morphotropic phase boundary (MPB), near the composition Zr : Ti = 52 : 48, where a tetragonal distortion (from the PbTiO3 side) of the perovskite cell changes for a rhombohedral one in the course of varying the Zr:Ti compositional ratio. Among other factors influencing the properties of PZT are dopant composition and concentration, nonstoichiometry, density and porosity of sintered ceramic specimens, grain and crystallite sizes, method of production and type of raw materials etc.
During the last decades, the properties of nanocrystalline PZT powders and consolidated bulk nanostructured materials attract increasing interest of researchers. Many techniques have been developed to produce nanocrystalline perovskite oxides: high-energy ball milling [2], laser deposition [3], various alternative wet-chemistry-based procedures such as hydrothermal methods, sol-gel processes, co-precipitation [4–8], thermal decomposition of organometallic, in particular oxalate, precursors [9–12]. The latter seems to have the best potential of adjusting the exact stoichiometry of a material.
More than 50 years after the pioneer work by W.Clabaugh [13] the details of chemical transformations and the nature of amorphous intermediate products in the process of decomposition of oxalate complexes remain disputable. It was shown that the temperature interval and the yield of fine-grained perovskite phase from decomposed oxalate precursor depend on heating rate in polythermic regime [14].
In the present paper, we report results of thermogravimetric and XRD study of thermal decomposition of oxalate precursor of morphotropic solid solution Рb(Zr0.52Ti0.48)O3.
Experimental
Reagent grade titanium tetrachloride TiCl4, zirconium oxychloride ZrOCl2·8H2O, lead nitrate Pb(NO3)2, oxalic acid H2C2O4·2H2O, ammonia NH3 25 % water solution and bidistilled water were used as starting materials to precipitate a PZT oxalate precursor.
First, titanium and zirconium hydroxides were precipitated with NH3 water solution (4.4–4.5 M) in a required proportion from chloride solution mixture (2.0–2.1 M):
0,48 TiCl4 + 0,52 ZrOCl2 + 2,96 NH3 + 2,48 H2O → H2(Ti0,48Zr0,52)O3↓ + 2,96 NH4Cl.
Wet residue (85–90 % H2O) was washed off from Cl- ions (test with AgNO3) on a Buchner funnel under reduced pressure. Then the residue was repulped in distilled water (S:L = 2:1) heated to 60 °C and dissolved in 2M oxalic acid with following neutralization to pH = 2.5:
H2(Ti0,48Zr0,52)O3 + 3 H2C2O4 → H2[(Ti0,48Zr0,52)(C2O4)3] + 3 H2O
H2[(Ti0,48Zr0,52)(C2O4)3] + 2 NH3 → (NH4)2[(Ti0,48Zr0,52)(C2O4)3].
After filtering, 1.5 M Pb(NO3)2 solution was added at intensive agitation at 80 °C keeping the value of pH in the interval of 4–5:
(NH4)2[(Ti0,48Zr0,52)(C2O4)3] + Pb(NO3)2 + 4 H2O → 2 NH4NO3 + Pb[(Ti0,48Zr0,52)(C2O4)3]·4H2O↓
The residue was filtered and washed off on a suction filter and then obtained PZT precursor – lead trioxalatozirconate–titanate tetrahydrate – was dryed at 120 °C and reduced pressure of 0.3 atm.
According to chemical analysis, the composition of dried precursor was Pb[(Ti0,48Zr0,52)(C2O4)3]·3.76H2O.
Thermogravimetric analysis was performed using a computerized original installation based on AS 220/C electronic balance (RADWAG, Poland).
Phase composition of samples after heat treatment at different temperatures was studied by X-ray powder diffraction (XRD). XRD data were collected on a DRON-3 diffractometer using Ni-filtered Cu Kα radiation at scanning rates 2° (2θ)/min.
Results and discussion
PZT perovskite phase is obtained by thermal decomposition of synthesized oxalate precursor. To find optimal conditions of heat treatment for this process, it is important to know the consequence and temperature intervals of chemical transformations during the precursor thermal decomposition.
In Fig. 1, the results of thermogravimetric study of synthesized precursor are shown. The decrease in the sample’s mass (%) is monitored as a function of temperature at the heating rate of 9 °C/min. As seen from these data, the loss of water molecules takes place before 200 °C, the decomposition rate increases sharply after the loss of first of three oxalate groups at about 250 °C and the loss of all oxalate groups is practically complete at 340 °C. Further heating up to 900 °C results in a very slow and small (< 2 %) mass loss, possibly due to PbO evaporation.
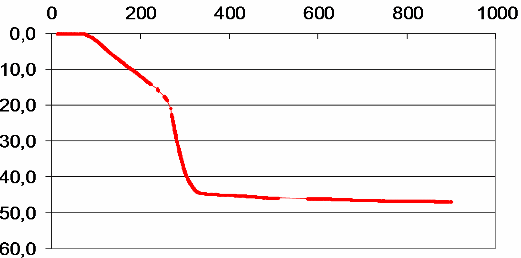
Fig. 1 – Weight loss (%) of PZT oxalate precursor as a function of temperature. Heating rate 9 °C/min.
Table 1. Results of XRD phase analysis
| Temperature, °C | Phase composition |
|---|---|
| 250–300 | Amorphous state |
| 350 | Onset of PbO crystallization |
| 400 | Mixture of weakly crystalline PbO (red and yellow) |
| 450 | Main crystalline phase is PbO (red), considerable amount of PbO (yellow),a lesser of amount of PbZrO3. |
| 500 | Large amounts of PbZrO3 and PbO (red), a considerable amount of PbO (yellow), a lesser amount of PbTiO3. |
| 550 | Large amounts of PbZrO3 and PbTiO3, considerable amount ofPbO (red and yellow), a lesser amount ofZrO2 and perhaps TiO2 (rutile) |
| 600 | Solid solutions based on PbZrO3 and PbTiO3, lesser amounts of PbO (red) and ZrO2. |
| 600–800 | The phase compositon of samples fired in this temperature interval changes slow with increasing temperature. Large amount of PbZrO3 and a lesser amount of PbTiO3. Considerable amount of PbO (yellow). PbO (red) transforms into yellow modification. |
To study phase composition of intermediate products of PZT oxalate precursor decomposition, weighed portions (≈10 g) of precursor were placed into the electric oven heated to a predetermined temperature and, after dwelling for 40 min, cooled to room temperature in air.
XRD diffraction patterns of heat treated samples are shown in Fig. 2. After treatment at 250–300 °C the samples are in amorphous state in X-rays, although the absorption maximum shifts to large angles (smaller interplanar distances). At 350 °C the onset of PbO crystallization is observed. The reflections of a perovskite phase appear at 400 °C and become absolutely clear at 450 °C. At 550 °C dominant amounts of PbZrO3 and PbTiO3, considerable amount of PbO (red and yellow), a lesser amount of ZrO2 and perhaps TiO2 (rutile) are present. Further increase in temperature results in a decrease of amounts of uncombined simple oxides, PbZrO3 and PbTiO3 are dominant phases, but they still do not form a homogeneous solid solution even at 800 °C. The phase compositon of samples fired in the 600–800 °C temperature interval changes rather slow with increasing temperature.
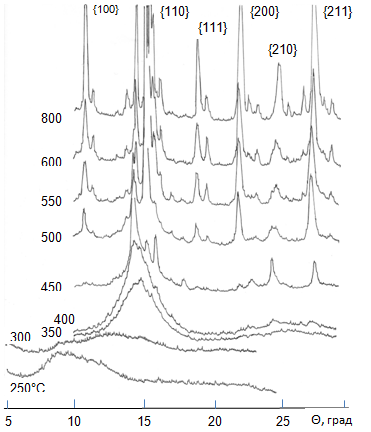
Fig. 2 – XRD patterns taken from products of thermal decomposition of PZT oxalate precursor at different temperatures for 40 min.
The main difficulty is a very slow homogenization of perovskite phases into PZT solid solution under discussed regimes of heat treatment. There is antagonism between the rate of solid-state reactions between simple oxides formed in the process of oxalate precursor decomposition and, from the other hand, the rate of particle growth for these oxides.
To work out this problem and find way to synthesize PZT from oxalate precursor at sufficiently low temperatures, we studied the possibilities of other heat- treatment schedules. It seemed interesting to try first decompose precursor at the lowest temperature leading to practically complete removal of oxalate ligands and then to increase temperature quickly to continue and complete solid-state reaction of PZT formation while the particles did not grow much.
Taking into account the results of XRD and TG studies the following heat treatment schedule was designed. A weighed portion of dried precursor was heated to 380 °C for the period of half an hour, dwelled at this temperature for 30 min. Then it was cooled to room ambient and pressed into pellets at 50 atm, these were placed again into the oven at 380 °C and finally quickly transferred into another oven already heated to 800 °C and dwelled for 40 min.
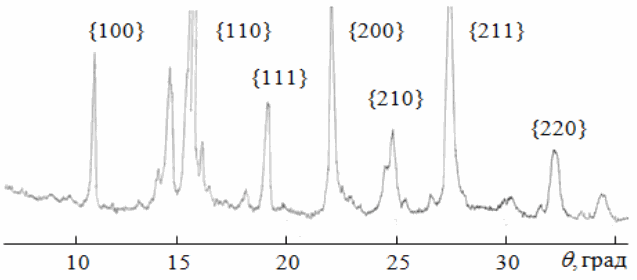
Fig. 3 – XRD pattern of product of precursor treatment according to a two-stage regime: 380 °C 40 min + 800 °C 40 min.
In Fig. 3 XRD pattern is presented for the material heat treated according to this schedule. The pattern shows reflections from PZT solid solution with elementary cell a = 4.10 Å. Also, reflections from excess PbO are visible. This result demonstrates possibility to synthesize a homogenious PZT solid solution from oxalate precursor at temperatures as low as 800 °C.
References
- B. Jaffe, R. S. Roth, and S. Marzullo Piezoelectric properties of lead zirconate – lead titanate solid-solution ceramics // J.Appl.Phys. – 1954. – V. 25. – No. 6. – P. 809–810.
- B. Praveenkumar, G. Sreenivasalu, H. H. Kumar, D. K. Kharat, M. Balasubramanian, B. S. Murty Size effect studies on nanocrystalline Pb(Zr0.53Ti0.47)O3 synthesized by mechanical activation route // Mater.Chem.Phys. – 2009. – V. 117. – P. 338–342.
- F. Craciun, M. Dinescu, P. Verardi, C. Galassi Pulsed laser deposition of nanocrystalline lead zirconate titanate thin films // Nanotechnology. – 1999. – V. 10. – P. 81–85.
- Q. F. Zhou, H. L. W. Chan, C. L. Choy Nanocrystalline powders and fibers of lead zirconate titanate prepared by the sol-gel process // J. Mater. Process. Technol. – 1997. – V. 63. – P. 281–285.
- Y. Faheem, M. Shoaib Sol-gel processing and characterization of phase-pure lead zirconate titanate nano-powders // J. Am. Ceram. Soc. – 2006. – V. 89. – No. 6. – P. 2034–2037.
- J. F. Meng, R. S. Katiyar, G. T. Zou, X. H. Wang Raman phonon modes and ferroelectric phase transitions in nanocrystalline lead zirconate titanate // Phys. Stat. Sol. (a) – 1997. – V. 164. – P. 851–862.
- G. Garnweitner, J. Hentschel, M. Antonietti, M. Niederberger Nonaqueous synthesis of amorphous powder precursors for nanocrystalline PbTiO3, Pb(Zr,Ti)O3, and PbZrO3 // Chem. Mater. – 2005. – V. 17. – P. 4594–4599.
- W. Zhu, Z. Wang, C. Zhao, O. K. Tan, H. H. Hng Low temperature processing of nanocrystalline lead zirconate titanate (PZT) thick films and ceramics by a modified sol-gel route // Jpn. J. Appl. Phys. – 2002. – V. 41. – P. 6969–6975.
- A. Banerjee, S. Bose Free-standing lead zirconate titanate nanoparticles: low-temperature synthesis and densification // Chem. Mater. – 2004. – V. 16. – P. 5610–5615.
- S. Roy, S. Bysakh, J. Subrahmanyam Metastable face-centered cubic lead zirconate titanate (PZT) and lead lanthanum zirconate titanate (PLZT) nanocrystals synthesized by auto-ignition of metal-polymer gel // J. Mater.Res. – 2008. – V. 23. – No. 3. – P. 719–724.
- M. S. Dash, J. Bera, S. Ghosh Study on phase formation and sintering kinetics of BaTi0.6Zr0.4O3 powder synthesized through modified chemical route // Alloys and Compounds. – 2007. – V. 430. – P. 212–216.
- R. A. Das, A. Pathak, P. Pramanik Low-temperature preparation of nanocrystalline lead zirconate titanate and lead lanthanum zirconate titanate powders using triethanolamine // J. Am. Ceram. Soc. – 1998. – V. 81. – No. 12. – P. 3357–3360.
- W. S. Clabaugh / W.vS. Clabaugh, E. M. Swiggard, R. Gilchrist // J. Res. Natl. Bur. Std. – 1956. – Vol. 56, No. 5. – P. 289–293.
- Рагуля А. В. Синтез и спекание нанокристаллического порошка титаната бария в неизотермических условиях. IV. Электронно-микроскопическое исследование эволюции морфологии порошков титаната бария / А.В. Рагуля, О.О. Васылькив, В.В. Скороход, Н.В. Даниленко // Порошковая металлургия. – 1998. – № 3/4. – С. 12–20.