Abstract
Contents
- Introduction
- 1 Theme urgency
- 2 Goal and tasks of the research
- 3 Summary of research and results
- 3.1 Selecting inhibitors for the permissible emulsion explosives
- 3.2 The development of the basic composition of the emulsion permissible explosives
- 3.3 Calculation of energy and permissible characteristics of the basic composition of the permissible emulsion explosives
- Conclusion
- References
Introduction
Drilling and blasting are among the main technological processes of mining. Therefore they have a significant impact on the state of industrial and environmental safety of the mining enterprise, and determine the cost-effectiveness of its work [1].
But at the same time drilling and blasting (DB) is a source of increased danger. Directly on the blasting operations accounted 0,7 % of fatal accidents. Especially dangerous possible consequences of an explosion. On average, in 20 % of explosions methane-air and dust-air mixtures, flares and burning methane and in 5 % of exogenous fires the reason is blasting [2]. In order to prevent explosions of methane-air and dust-air mixtures used permissible explosives. For the different geological conditions and the degree of danger workings of gas and dust are used explosives of different classes.
In addition to the factors instant action, explosions are characterized by multiple processes impact on the environment, one of which the pollution of air space, soil and water blasting products [3]. In applying the permissible explosives in the geological conditions of occurrence of layers of rocks with a high temperature (<50 °C) and faces poorly ventilated mines, arise a toxic effect on the human organism not only the products of the explosion of explosives, but also highly toxic ingredients in their composition. So the composition contained in the permissible explosives nitroesters and TNT, which, according to GOST 12.1.005–88 and GOST 12.1.007–76 refer to I and II class of hazard for toxic effects on the human organism, making these explosives environmentally hazardous [4]. Developing environmentally friendly permissible explosives, wich not containing in its composition of toxic components, will solve this problem.
1 Theme urgency
The introduction of emulsion explosives led to the solution of several important problems:
- Emulsion explosives have low sensitivity to mechanical stress, thus improving the safety of blasting.
- Emulsion explosives do not contain toxic components, which makes them environmentally friendly explosives.
However, to date, all industrial emulsion explosives manufactured in Ukraine, do not have the permissible features that complicates and limits their application in mines dangerous on gas and dust. Therefore, the creation of emulsion explosives, having a permissible level that corresponds to the class IV and V, is an urgent task.
2 Goal and tasks of the research
The goal of research in this paper is to examine the possibility of ensuring the permissible properties, through inject in the composition of emulsion explosives methane flame retardants. To achieve this objective the need to solve the following tasks:
- Conduct a literature review of existing methane flame retardants.
- Choose the most effective and safe inhibitors in terms of toxicity.
- Determine the theoretical amount of inhibitor in the emulsion explosives composition to impart safety permissible at the level of class IV–V.
- Conduct research on the compatibility of the components in the oxidizer solution of the emulsion explosives.
- Calculate the final composition of emulsion permissible explosives class IV.
- Calculate the final composition of emulsion permissible explosives class V.
3 Summary of research and results
3.1 Selecting inhibitors for the permissible emulsion explosives
In industrial explosives class IV as inhibitor used alkali metal halides. According to research conducted previously [5,6], the alkali metal halides are the most active negative catalysts.
Methane oxidation by atmospheric oxygen is described by the gross reaction:
CH4 + O2 → CO2 + 2H2O
In general, the oxidation of hydrocarbons is a complex radical chain reaction with degenerate chain branching. The main chain of V. N. Kondratyev [7], is preferably a reaction involving hydrocarbon and hydroxyl radicals, and singlet oxygen:
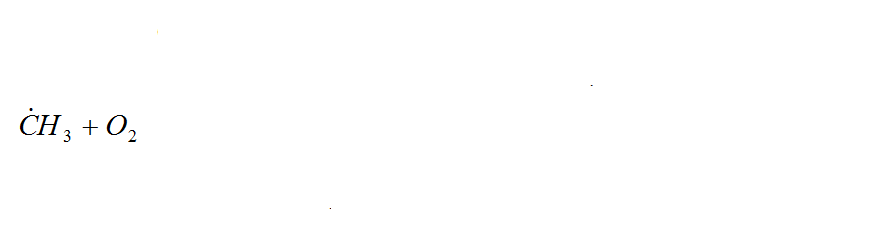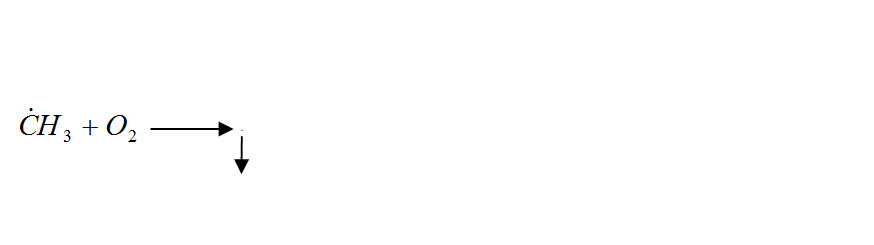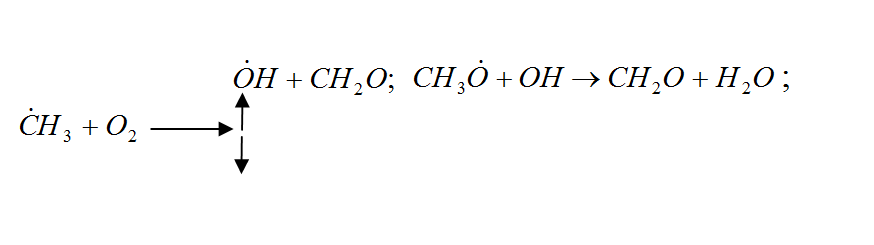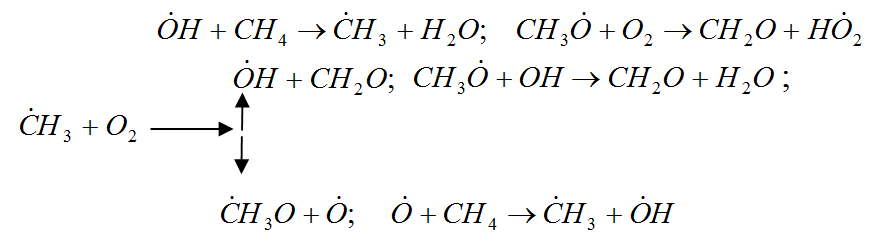
Figure 1 – Radical chain reaction of methane oxidation
(animation: 6 frames, 8 cycles of repeating, 23 kilobytes)
L. Dolan and P. Dempcter [8] measured the flame retardant ability of a number of salts, insufflation powders in the heated container with the gas, and we received a number of descending flame retardant ability: KF, KI, NaAlF3, Nacl, Na2SiF4, KCl. Murat T. [9] established the following series efficiency of flame arresters (in descending order): NaCl, KCl, K2CO3, Na2CO3, CaCl2, NH4Cl. But not all halogens alkali metal may be incorporated into safety explosives, for example, the alkali metal compound with fluorine and bromine are toxic.
As can be seen from the foregoing, quite active, environmentally friendly, cheap and not scarce flash suppressor is NaCl.
MakSRI studies have shown that to ensure the safety properties of the emulsion explosives at the level of class IV, the content of the flame arrester (given the presence of 10 % water, which is also a flame arrester) in their composition should be at least 10–12 %. The solubility of NaCl is quite low – 35,9 g / 100 g water at 20 °C, and with increasing temperature remains virtually unchanged. A water content in the emulsion explosives composition does not exceed 10–12 %, from the viewpoint of maintaining high power characteristics. An increase the water content in the emulsion explosive composition by 1 % to reduce energy composition on ~2,5 % and entails a reduction of the percentage of the oxidizer phase in the composition. Thus, increasing the water content in the emulsion explosives composition is undesirable, from the viewpoint of loss of useful power and efficiency characteristics of the composition [10].
The maximum amount of NaCl that we can dissolve at 10–12 % of water is about 3 % in relation to 1 kg of explosive. Adding a solid fraction reduces the detonation characteristics of explosives. As the flame arrester may also be used such salts, as KCl and CaCl2, having higher values of solubility. One of the key questions of this work, was a question about the possibility of using the combined arrester, consisting of NaCl, KCl и CaCl2, in the composition of emulsion explosives. Our studies have shown that these salts do not have a mutual influence on the solubility of each salt alone, which allows the introduction in the composition of the emulsion explosive desired amount of arrester.
3.2 The development of the basic composition of the emulsion permissible explosive
The most common oxidizing agent is ammonium nitrate. Use as a dispersed phase solution of ammonium nitrate is associated
with certain technological difficulties. Since the 90 % ammonium nitrate solution has a crystallization temperature of 160 °C.
Preparation of the inverse emulsion and maintaining such a high temperature in the production conditions pits
technologically difficult and not safe, and often impossible [11]. Considering the number of components that could reduce
the crystallization temperature, the choice was made in favor of a mixture of ammonium nitrate and calcium. As the fuel
phase in the basic composition used industrial oil. Emulsifier – Lubrizol
.
Designed basic composition is shown in table 1.
| Components | |||||||
|---|---|---|---|---|---|---|---|
| Fuel phase | KCl | NaCl | CaCl2 | Water | NH4NO3 | Ca(NO3)2 | |
| Contents, % | 6 | 3,2 | 3,3 | 6,5 | 9,4 | 51,5 | 20,1 |
3.3 Calculation of energy and permissible characteristics of the basic composition of the permissible emulsion explosives
Equation explosive transformation of the basic composition will be as follows:
0,177C24H50 + 0,43KCl + 0,56NaCl + 0,58CaCl2 + 5,22H20 + 6,44NH4NO3 + 1,22Ca(NO3)2 → 0,43KCl + 0,56NaCl + 0,58CaCl2 + 1,22CaCO3 + 22,5H20 + 2,67CO2 + 0,36CO
Calculation of exothermicity of the explosion will be carried by the formula:
Q = Qpr. + Qex.
Q – exothermicity of explosion, kcal/kg;
Qpr. – heat of formation of explosion products, kcal/kg;
Qex. – heat of formation of explosive, kcal/kg.
The value of the heats of formation of components of explosives and explosive products are taken from the source [5]. The resulting value Q = 714 kcal/kg or 2991 кJ/kg.
The oxygen balance of composition is calculated as follows:
Ox.b. = Σ Xi× ox.b.i
Ox.b. – oxygen balance of explosive, %;
Хi – the mass fraction of component i, kg/kg;
ox.b.i – oxygen balance of component i, %.
The oxygen balance of explosive equals –0,66. It is known that when a small negative oxygen balance in the course of explosive transformation released the least amount of toxic gas (carbon monoxide and nitrogen oxides). The resulting calculated value is the oxygen balance in the above range, which provides a minimum emission of toxic gas in the explosion of explosives, that is, it provides environmental safety.
Calculation of permissible features will be carried by the method proposed B. I. Vaynshteynom [12]. As the quantitative characteristics of the permissible level will be calculated mass of the explosive charge, which in the given conditions is observed 50 % of ignitions, by the empirical relationships:
- for the charges in the channel mortars:
m50 = 1,41 × 1020 × Q-5 × β-1,83
m50 – mass of the explosive charge, which in the given conditions is observed 50 % of ignitions, g;
Q – exothermicity of explosion, kJ/kg – 2991.
β = D / (0,063 × ρ-0,7 × Q0,5)
D – velocity of detonation, km/s;
ρ – density of the explosive at which detonation of velocity measured, g/sm3 – 1,2;
To perform calculations take the value of the detonation of velocity equal to 4 km/s (certain for the similarities by the composition emulsion explosives).
The resulting value m50 – 566 g.
СоAccording to GOST 7140, when testing the permissible explosives IV class, in the test drift produced 20 explosions of the charge weight 300 g. It is allowed to obtain up to 50 % ignition (9 ignitions of 20 experiments). Therefore m50 it should be more than 300 g.
The resulting calculated value is 2 times greater than the requirements of GOST 7140, which makes it possible to say with certainty that the present explosive has the necessary permissible properties.
Conclusion
From the foregoing, it follows that the use of explosives containing TNT and nitrate esters, causes significant damage to the environment. To improve the ecological safety need to use eco-friendly compositions of explosives based on emulsions.
To ensure the safety properties of emulsion explosives is necessary to apply a combination flash suppressor, allowing you to enter into the required quantity of explosive flame arrester.
The results of research in the future will be the basis for the development of the industrial design of the permissible emulsion explosives.
This master's work is not completed yet. Final completion: December 2015. The full text of the work and materials on the topic can be obtained from the author or his head after this date.
References
- Захаренков Е. И. Состояние взрывного дела в Украине. Государственный надзор в сфере обращения со взрывчатыми материалами промышленного назначения / Е. И. Захаренков // Информационный Бюллетень УСИВ. – 2010. – № 4. – С. 4–8. [Электронный ресурс]. – Режим доступа: http://usiv.com.ua.
- Александров С. М. Охорона праці у вугільній промисловості: Учбовий посібник для студентів гірничих спеціальностей вищих учбових закладів / C. М. Александров, Ю. Ф. Булгаков, В. В. Яйло. – Д.: РІА ДонНТУ, 2004. – 480 c.
- Корнет В. В. К вопросу использования взрывчатых веществ на горнорудных предприятиях Кривбасса / В. В. Корнет, О. В. Прохода, С. М. Чухарев // Вісник Кременчуцького державного політехнічного університету імені Михайла Остроградського. – 2014. – Вип. 4/2014 (87). – С. 113–117. [Электронный ресурс]. – Режим доступа: http://www.kdu.edu.ua.
- Калякин С. А. Обоснование технических требований к экологически чистым предохранительным ВВ / С. А. Калякин, К. Н. Лабинский, Е. В. Терентьева // Вісник Кременчуцького державного політехнічного університету імені Михайла Остроградського. – 2014. – Вип. 6/2009 (59). Частина 1 – С. 169–174. [Электронный ресурс]. – Режим доступа: http://www.kdu.edu.ua.
- Дубнов Л. В. Промышленные взрывчатые вещества / Л. В. Дубнов, Н. С. Бахаревич, А. И. Романов. – М.: Недра, 1988. – 358 с.
- Светлов Б. Я. Теория и свойства промышленных взрывчатых веществ / Б. Я. Светлов, Н. Е. Яременко. – М.: Недра, 1973. – 208 с.
- Кондратьев В. Н. О теории горения углеводородов / В. Н. Кондратьев // «Журнал физической химии». – 1946. – № 4–5. – C. 345–354.
- Dolan L. E., Dempster P. B. Journ. of applied Chemistry. V. 5, part 9. – 1955. – pр. 510–517.
- Murata T. Диффузионная теория горения метана / T. Murata // Japan Sci. Rev. Engang Dci., 2. – 1952. – № 4. – pр. 421–427.
- Тимофеева А. М. Исследование возможностей обеспечения предохранительных свойств ЭВВ / А. М. Тимофеева, Ю. В. Манжос // Инновационные перспективы Донбасса. Материалы Международной научно-практической конференции. – Донецк – 2015.
- Стрилец А. П. Выбор состава и концентрации раствора окислителя для приготовления эмульсионных взрывчатых веществ типа украинит / А. П. Стрилец // Сборник научных трудов НГА Украины – 2009. – № 12, Том 2. – C. 145–149.
- Технические требования к патронированным ВВ IV и V. Методика предварительной (аналитической) оценки основных свойств предохранительных ВВ. – Изд. МакНИИ, Макеевка – Донбасс, 1983. – 88 с.
