Abstract
Content
Introduction
Along with the development of electric power systems, the growth of renewable energy capacities the problem of energy accumulation and storage becomes more acute.
One of the ways to save electricity is to use chemical current sources — batteries. There are two types of the most widely used types of batteries — lead–acid (starter batteries on vehicles, backup and emergency sources of energy) and lithium–ion (modern household appliances, electric cars, electric power industry).
1. Lithium–ion battery
The first lithium–ion battery was released by Sony in 1991. In the first lithium-ion batteries, lithium was used as the material of the anode,
which, due to frequent charge–discharge, led to the growth of sponge-like structures of this metal — dendrites.
This could lead to the closure of the electrodes, which caused explosions and fires. The problem was solved by using lithium–rich graphite instead
of pure lithium [1]. Graphite inhibits the growth of dendrites as a result of intense lithium sedimentation, but this leads to a significant
reduction in battery capacity. When a battery is discharged, lithium ions are extracted from the carbon material of the negative electrode
and accumulated in the form of lithium oxide on the positive electrode. For charging the processes goes in the opposite direction.
Consequently, there is no metallic (zero–valent) lithium throughout the system, and the charge and discharge processes are reduced
to the transfer of lithium ions from one electrode to another. Therefore, these batteries are called lithium–ion
.
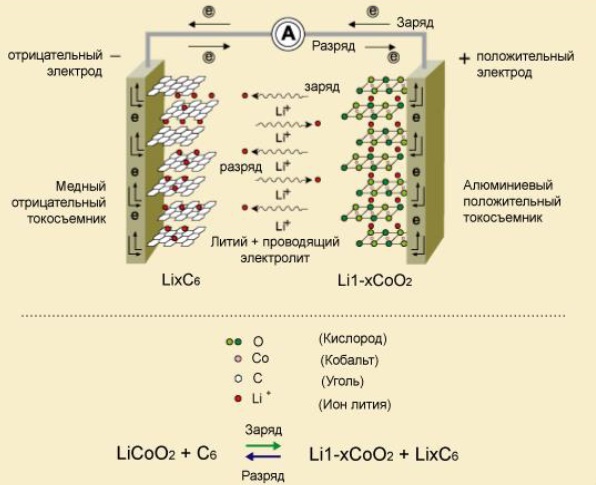
Picture 1 — The principle of operation of the Li–ion battery
Lithium–ion batteries have protection against internal short circuits, in some cases there is also protection against external short circuits.
The advantages of lithium–ion batteries are: high energy density, low self-discharge, ease of maintenance.
The advantages also include the absence of a memory effect
, manifested in a reversible decrease in capacity due to deviations
of the recommended charge-discharge mode of the battery. [2]
The disadvantages of lithium–ion batteries: sensitivity to overcharging and re–discharging, so they must have charge and discharge limiters; capacity decrease at low temperatures; relatively fast aging. The life cycle of such battery is affected by the depth of its discharge before the next charging, as well as charging by currents higher than those set by the manufacturer. Batteries are also sensitive to charging voltage. Battery overheating is possible. Lithium batteries age even when not in use. For 2 years, the battery loses about 4% of capacity. [2]
In order to increase the capacity of lithium batteries was started development of lithium–air batteries.
2. Lithium–air batteries
For the new batteries it was decided to stop using the positive electrode as such. The current–forming reaction is the direct interaction of lithium with atmospheric oxygen, which use as a cathode can lead to energy densities 5–10 times higher than they are currently obtained [3]. Lithium cobalt is not used in lithium–air batteries that allows them to be significantly lighter compared to existing batteries. The main disadvantages of lithium–air batteries are fast run–out and low energy efficiency.
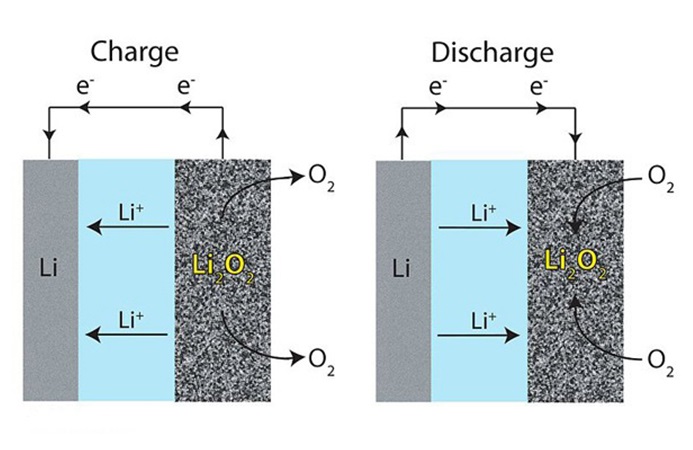
Picture 2 — The principle of operation of the lithium–air battery
The development of lithium–oxygen batteries promises to solve this problem. They use nanoparticles containing lithium and oxygen. At the same time, unlike lithium–air batteries, oxygen, when states change, is stored inside the particle and does not return to the gas phase. This reduces energy loss and increases battery life. Also, these batteries, unlike lithium–air batteries, are protected from overcharging and do not deteriorate in contact with moisture and carbon dioxide. But for now, this is only a laboratory prototype (research and development is conducted at the University of Cambridge) [4].
Conclusion
In conclusion, we can draw the following conclusions.
Currently, one of the most widely used type are lithium–ion batteries, however, they have a number of drawbacks, such as sensitivity to ambient temperature, sensitivity to overcharging and over discharge, as well as sensitivity to charging voltage. Lithium–air batteries allow you to increase capacity, but after a certain number of cycles, they quickly run–out, and also in the process of work they release too much energy and heat for nothing. New lithium–oxygen batteries are in the process of development, unlike lithium–air batteries, oxygen in them is stored inside the nanoparticle and does not return to the gas phase that reduces energy loss and extends battery life.
References
- Эволюция аккумуляторов: «Когда же мы забудем про розетки». [Электронный ресурс]. — Режим доступа:http://mediate-club.ru/publ/...
- Литий–ионные и литий–полимерные аккумуляторы iXBT (2001 г.)
- Вольтер С. Н., «Литий–воздушные аккумуляторы», 2013. [Электронный ресурс]. — Режим доступа: http://www.electra.com.ua...
- Science 30 Oct 2015: Tao Liu, Michal Leskes, Wanjing Yu, Amy J. Moore, Lina Zhou, Paul M. Bayley, Gunwoo Kim, Clare P. Grey: «Cycling Li–O2 batteries via LiOH formation and decomposition»
- Бояршинов Д. С., «Графеновый аккумулятор», 2017 [Электронный ресурс]. — Режим доступа: http://akbinfo.ru...
- Взрыв в мире аккмуляторов: «Графеновые батареи». [Электронный ресурс]. — Режим доступа: http://expertpost.ru...
- «Аккумуляторы которых нет». Новейшие разработки. [Электронный ресурс]. — Режим доступа: https://mediatek-club.ru...
- Васильев В. В. «Литий–ионные и литий–полимерные аккумуляторы»
- Хрусталев Д. А. «Аккумуляторы» Изумруд, 2003 г.— 224 с.
- Дьякова А. Ф. Энергетика сегодня и завтра. — М.: Энергоатомиздат, 1990. — 344 с.
