| ДонНТУ | Портал магистров | Биография | Автореферат | Библиотека | Ссылки | Отчет о поиске |
Nitration can be carried out either directly by introduction of the nitro group in place of a hydrogen atom or by adding it to a double bond, or else indirectly, by introducing into a compd a group which can readily be replaced by or converted to the nitro group.
The following nitrating agents are most frequently used in industry for the direct introduction of the nitro group:
For lab prepns, and occasionally in industrial use, more expensive nitrating agents may be employed, as for example solns of nitric acid in inert organic solvents (chlf, carbon tetrachloride, eth, nitromethane, etc), or a soln of nitric acid in phosphoric or acetic acids or in acetic anhydride, trifluoroacetic anhydride or trifluoro-methanesulfonic acid.
For nitrating on the lab scale, mixts of nitric acid esters or acyl nitrates, eg acetyl nitrate (CH3CONO3) and sulfuric acid may also be used.
Several lesser known nitrating agents, which have been used on a lab scale are metal nitrates in the presence of acetic acid or acetic anhydride, tetranitromethane and hexanitroethane in an alkaline medium, and nitroguanidine in soln in sulfuric acid, used for the nitration of aromatic amines and phenols.
The most common indirect nitration method, often used in nitrating phenols, consists of sulforating the compd and then replacing the sulfo group by a nitro group. The usual nitrating agent for these reactions is concd nitric acid.
Other indirect nitration methods applied on an industrial scale for nitrating phenols involve introducing a nitroso group into the phenol and then oxidizing it to the nitro group. Another method involves the oxidation of a primary amino group to the nitro group.
In exptl work, indirect methods of introducing nitro groups find wide application as, for example, the replacement of a halogen (iodine or bromine in an alkyl iodide or bromide) by the nitro group, by means of silver nitrite (the Victor Meyer reaction).
In aromatic compds, an amino group may be replaced by the nitro group by diazotization and reaction with nitric acid in the presence of cuprous salts (the Sandmeyer reaction). This method is used for lab work only and is described in standard textbooks on preparative organic chemistry.
The most widely used nitrating agents in the prepn of important military and commercial high expls are the mixed acids (MA) consisting of various mixts of HNO3 / H2SO4 / H2O. Consequently the remainder of this section will be devoted to a discussion of mixed acids. In view of the obvious importance of its use in the prepn of military expls, there is a US Military Specification for Mixed Acid (for use in nitration of explosives): MIL-A-50210(MU) (6 December 1968). The "Requirements" for mixed acid prescribed in this specification are:
The appropriate mixed acid compns for the nitration processes that produce militarily and industrially important expls will be described in Sections V & VI. Typical MA compns for aromatic nitrations contain 110 to 200% nitric acid over the stoichiometric requirement. For the nitration of toluene to MNT & DNT, a typical MA compn in round figures is: 30% HN03, 60% H2SO4, 10% H2O. For the nitration of DNT to TNT the MA contains no water and is approx: 20% HNO3, 80% H2SO4
Nitrate esters such as NG & EGDN are made using MA contg 30-70% HNO3, 35-70% H2SO4 and 0-10% H2O. The nitric acid content ot the MA is usually 20% in excess of stoichiometric
The ratio of the quantities of components of the nitrating acid (HN03 H2SO4 and H2O) is obviously important. The fact that water is formed during the nitration process, thus diluting the nitrating mixt, must be taken into consideration. Since sulfuric acid is the principal "dehydrating" component of MA, the amount of H2SO4 must be chosen in such a proportion that it can take up most of the matter formed during the nitration. Otherwise nitration might be incomplete. A commonly used measure of the effectiveness of the sulfuric acid in doing this is the DVS or dehydrating value of sulfuric acid. DVS is defined by:
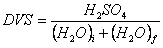
where (H2O)j is the initial concn of water in the nitrating mixt before nitrating and (H2O)f is the concn of water formed during nitration.
The DVS of a given MA should be as high as possible in order to obtain complete nitration. However, as discussed in the next section, compromises must often be made in order to minimize the solubility of the nitrated product in the spent acid, ie, the acid in equilibrium with the nitrated product, as well as the solubility of spent acid in the product.
Gillespie and Miller arranged various nitrating agents in order of increasing nitration effectiveness, namely:
| C2H5ONO2 | ethyl nitrate |
| HONO2 | nitric acid |
| CH3COONO2 | acetyl nitrate |
| NO3NO2 | nitric anhydride |
| ClNO2 | nitryl chloride |
| H2ONO2+ | nitracidium ion |
| NO2+ | nitronium ion |
According to Urbanski (Ref 74, Chapt 2), this order seems to require some alteration. For example, nitryl chloride has been found to be a definitely weaker nitrating agent than nitric acid and should have been placed before it. The nitronium ion, NO2+, occurs in many mixed acid compns
Acid compns for practical nitrations must be formulated in such a manner that the spent acid must:
It is obvious that the nitrated product must be separated from the acid in equil with it (spent acid). If the product and the spent acid form two immiscible liq phases, eg, NG, EGDN, or molten TNT, separation is effected by gravity or centrifuging. If the product and spent acid form a solid and a liq phase, eg PA, NC or PETN, separation is effected by centrifuging (PA & NC) or filtration (PETN). If the nitration is carried in the vapor phase (NM), separation is effected by distillation.
To keep product yield at a maximum it is important that the solubility of product in the spent acid be kept to a minimum. This also facilitates removal of traces of product from the spent acid so that it can be either fortified and reused, reused to make lower nitro compds, or neutralized and discharged as non-polluting waste material.
Traces of acid in the product almost always degrade product stability and in some cases can lead to self-ignition and expln. The usual methods of removing traces of spent acid is by washing with water and/or dil alkali solns followed by a water wash.
Typical nitrating acid and spent acid compns used in the manufacture of selected high expls are shown in Table 2.
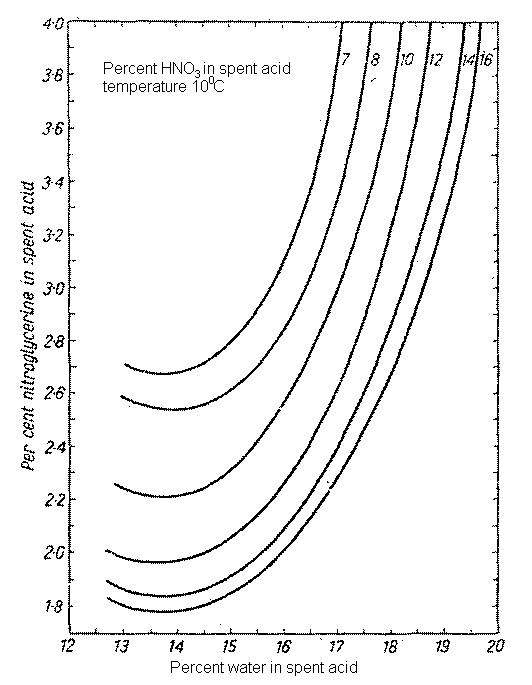
As illustrations of the loss of yield that can occur if spent acid compns are not adjusted to maintain minimum solubility of product, consider the data in Fig 1. These show a 2-3 fold increase in NG solubility in spent acid contg 7-16% HNO3 and 16-19% H2O over that dissolved in spent acid contg 16% HNO3 and 13-15% H2O.
Similarly, TNT is very soluble in cone H2SO4 as shown in Table 3, and considerably less soluble in spent acid contg small amts of nitric acid, as shown in Table 4. According to Orlova TNT is very soluble (100-800%) in conc HNO3.
It is also important to minimize the solubility of acid in the product. Fig 2 shows the solubility of HN03 in NG. The numbers at the right of the curves are the % HNO3 in the spent acid. The vertical line corresponds to the mole ratio of H2O / H2SO4 to form the monohydrate H2SO4.H2O. Note that the max HNO3 solubility for each curve occurs close to this vertical line. It is clear that both water content and HNO3 content of the spent acid should be kept low in order to minimize HNO3 solubility in the NG.
| Product | Nitrating acid (%) | Spent acid (%) |
| MNT (a) | 28/56/16 HNO3/ H2SO4 / H2O | 70/30 H2SO4 / H2O |
| DNT (a) | 28/64/8 HNO3/ H2SO4 / H2O | 2/76/22 HNO3/ H2SO4 / H2O |
| TNT (a) | 20/80 HNO3/ H2SO4 | 4/86/10 HNO3/ H2SO4 / H2O |
| TNT (b) | 8/90/2 HNO3/ H2SO4 / H2O | - |
| PA (a) (c) | 70/20/10 HNO3/ H2SO4 / H2O | 4/76/18/2 HNO3/ H2SO4 / H2O / other |
| NG (a) | 50/50 HNO3/ H2SO4 | 13/71/16 HNO3/ H2SO4 / H2O |
| NG (b) | 50/50 HNO3/ H2SO4 | - |
| DEGDN (a) | 65/35 HNO3/ H2SO4 | 29/45/22/4 HNO3/ H2SO4 / H2O / DEGDN |
| PETN (a) | 99/1 HNO3/ H2O | 30/70 HNO3/ H2O (d) |
| NC (a) | 22/68/9 HNO3/ H2SO4 / H2O | 19/70/11 HNO3/ H2SO4 / H2O |
| tetryl (a) | 78/6/16 HNO3/ H2SO4 / H2O | 1/0.5/82.5/16 HNO3/ NO2 / H2SO4 / H2O |
* - these are approximate "average" compns for several different processes
(a) - Batch nitration; (b) - Continuous nitration; (c) - Final step; (d) - Water is added to ppt the PETN
| Temperature, 0C | Concentration of H2SO4, % | ||||||
| 70 | 75 | 80 | 85 | 90 | 95 | 100 | |
| 0 | - | 0.3 | 0.4 | 0.6 | 2.0 | 3.5 | 13.0 |
| 10 | - | 0.3 | 0.45 | 0.75 | 2.2 | 4.0 | 13.5 |
| 20 | - | 0.3 | 0.5 | 0.85 | 2.5 | 4.8 | 15.0 |
| 25 | - | 0.32 | 0.55 | 0.95 | 2.6 | 5.2 | 15.5 |
| 30 | - | 0.35 | 0.6 | 1.0 | 2.7 | 6.0 | 16.5 |
| 40 | 0.2 | 0.4 | 0.65 | 1.3 | 3.0 | 7.0 | 18.0 |
| 50 | 0.2 | 0.45 | 0.70 | 1.7 | 3.5 | 8.5 | 21.0 |
| 60 | 0.22 | 0.50 | 1.0 | 2.3 | 5.2 | 11.0 | 24.8 |
| 70 | 0.35 | 0.7 | 1.6 | 3.3 | 7.0 | 13.5 | 29.0 |
| 80 | 0.6 | 1.3 | 2.4 | 4.8 | 10.0 | 18.0 | 26.5 |
| Composition of the mixrute, % | Solubility, % | ||||
| H2SO4 | HNO3 | H2O | at 20 0C | at 50 0C | at 70 0C |
| 60 | 0 | 40 | 0.20 | 0.52 | 0.70 |
| 80 | 0 | 20 | 0.59 | 1.25 | 2.07 |
| 90 | 0 | 10 | 2.55 | 4.70 | 7.63 |
| 60 | 1 | 39 | 0.22 | 0.41 | 0.62 |
| 80 | 1 | 19 | 0.55 | 1.08 | 1.68 |
| 90 | 1 | 9 | 1.85 | 4.35 | 7.49 |
| 60 | 5 | 35 | 0.25 | 0.55 | 1.23 |
| 80 | 5 | 15 | 0.73 | 1.48 | 1.85 |
| 90 | 5 | 5 | 1.76 | 4.49 | 7.53 |
| ДонНТУ | Портал магистров | Биография | Автореферат | Библиотека | Ссылки | Отчет о поиске |