| ДонНТУ | Портал магистров | Биография | Автореферат | Библиотека | Ссылки | Отчет о поиске |
There are two main theories concerning the nitration of hydrocarbons by means of the nitrating agents described above.
The first theory assumes a two-stage reaction with an addition reaction as the first stage and the second assumes that nitration is a double exchange reaction. At present the second theory has more adherents, since it is based on more recent experimental data.
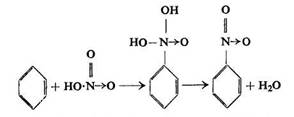
According to Michael, on nitrating aromatic hydrocarbons an intermediate addition product is formed, which has one hydrocarbon hydrogen atom attached to one oxygen atom of nitric acid, and a carbon atom of the aromatic ring directly attached to the nitrogen atom of the acid. The "aldol" formed gives off water in the presence of an excess of nitric or sulphuric acid. The mechanism was depicted by the author as follows:

Likewise the mechanism of nitration of an aromatic hydrocarbon with nitrosulphuric acid SO2(OH)ONO2was formulated by Michael as:
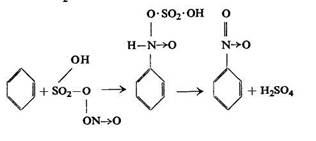
Here it is not water but sulphuric acid which is given off.
This view Michael confirmed in 1935 when he emphasized that in a molecule of nitric acid the dominating factor, which facilitated the reaction, was the affinity of oxygen for hydrogen and of nitrogen for the aryl group.
Hence the facility of the transformation:
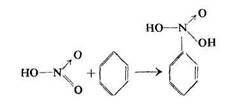
However, Giersbach and Kessler supposed that the initial step in the nitration reaction was the addition of two nitric acid molecules to a benzene molecule.
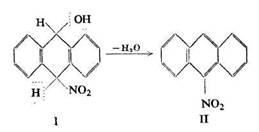
Experimental evidence of the possibility of the formation of products from aromatic hydrocarbons by the addition of nitric acid was provided by Meisen-heimer. He found that with anthracene the nitric acid molecule attached itself to the 9 and 10 carbons (of aliphatic character), yielding the product I, which in the presence of sodium hydroxide or acetic anhydride gave off water to form meso-nitroanthracene (II):
It has also been found that certain aldehydes, ketones and carboxylic acids esters form well defined products with nitric acid. For example, bemzaldehyde with 60% nitric acid gives a colourless, unstable oil, and cinna-mic aldehyde forms fairly stable white crystals, melting at 60-61°C, with 65% nitric acid. Acetophenone, benzophenone, fiuorenone, phenanthrenoquinone and camphor give similar addition products.
They are converted to nitro compounds under the influence of nitric acid or acetic anhydride and are decomposed by water to give the initial components.
According to T. Urbanski and Hofman the ionic oxonium salt structure can be attributed to these compounds:
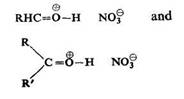
This was based on infra-red absorption spectra which show frequencies of the oxonium ion bond (O-H, ca. 2600 cm-1) and of the nitrate ion.
Houben gives the following sequence of transformations for "benzaldehyde nitrate":
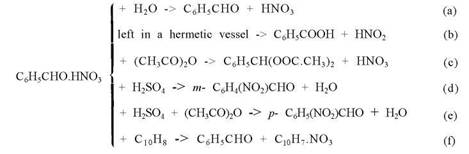
An interesting point is the influence of the compounds which react with an addition compound (reactions d and f) on the position of the nitro group introduced.
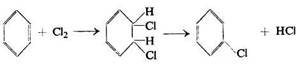
Following Thiele's view that any aromatic substitution is preceded by the formation of an addition product Holleman suggested in 1910 that the reaction of nitration, like that of chlorination, consisted in addition, followed by splitting off, according to the following scheme for chlorination:
A similar scheme for the nitration process was given by Reddelien who expressed the view that nitration of aromatic hydrocarbons with mixtures of nitric and sulphuric acids gave addition products, e.g.
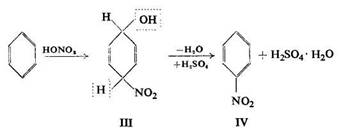
The product (III) undergoes decomposition, the group OH being attached to H2SO4 as H2O (IV). The addition product is hydrolysed by water, and mono- or polynitro compounds are formed.
Mainly on the basis of Holleman's hypothesis and studies on the nitration of olefins, Wieland assumed the addition of a nitric acid molecule to the double bond, resulting in the formation of a cyclohexadiene derivative (V), followed by the loss of a water molecule:
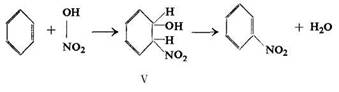
The addition of a nitric acid molecule to the double bond was first studied by Kekule, who obtained an oily, explosive product on treating ethylene with concentrated nitric acid. Wieland and Anschiitz believed the reaction to proceed principally according to the eqns. (8) and (9).
In support of this theory Wieland reported the results of his investigations, carried out in co-operation with Sakellarios, where two products (VI) and (VII) were obtained in the reaction of ethylene with nitric acid:
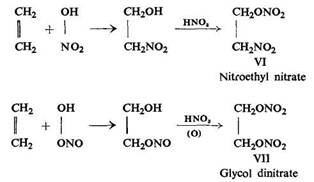
In both reactions products were formed which resulted from addition (in the first stage of the reaction) of a nitric acid molecule to the double bond.
Wieland's theory was criticized. Michael and Carlson called in question Wieland's view and proposed a different mechanism:
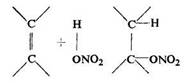
since they found that at temperatures below 0°C concentrated nitric acid adds to defines, such as isobutylene, trimethylethylene, to yield the nitric esters of the corresponding alcohols. Other objections to the Wieland theory were also put forward by Topchiyev:
1. The cyclohexadiene derivative (V), formed by addition of a nitric acid molecule, is very unstable and it is difficult to speak about a definite direction of the decomposition reaction of the compound V.
2. Against the theory of the similarity of the processes of attaching HN03 and Br2 to the double bond is the fact that molecules are attached with different rate. Bromine is attached only with great difficulty (without a catalyst). On the contrary, nitration is very easy to carry out.
Taking this into account, Tronov and Nametkin and Zabrodina advanced another idea, similar to Michael's initial hypothesis. Thus on the basis of Giersbach and Kessler's experiments Tronov inferred that one of the two HNO3 molecules reacting with one molecule of benzene acts as a catalyst.
On the basis of Boedtker's experiments, who found that benzene was nitrated by ethyl nitrate in the presence of aluminium chloride, Tronov suggested the following mechanism for this process:
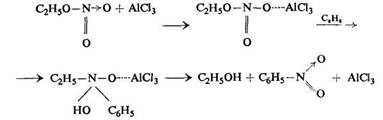
By analogy with this, Tronov gives the following plan for the general mechanism of nitration:
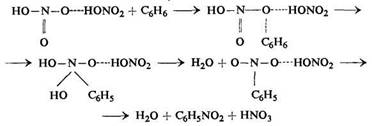
According to Schaarschmidt the mechanism of nitration with a mixture of nitric and sulphuric acids consists in the formation of nitric anhydride which becomes attached to the aromatic compound. The addition compound is unstable and decomposes, giving a nitro compound and nitric acid. The mechanism of nitration suggested by Schaarschmidt is:
Hetherington and Masson suggested that nitrobenzene can form complexes with H2SO4 and HNO3 and that the cation, C6H5NO2H+, of these complexes reacts with HNO3 to form dinitrobenzene:
H2SO4 + C6H5NO2 <=> C6H5NO2H+ + HSO4-
HNO3 + C6H5NO2 <=> C6H5NO2H+ + NO3-
C6H5NO2H+ + H2O <=> C6H5NO2 + H3O+
C6H5NO2H+ + HNO3 <=> C6H5(NO2)2 + H3O+
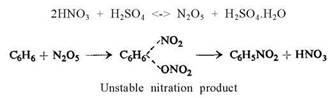
Lauer and Oda assumed the existence of nitracidium sulphate (according to Hantzsch) and suggested that the mechanism of nitration with a nitrating mixture is as follows:
A similar nitration mechanism was suggested by Vorozhtsov. He also assumed the formation of an addition product of the hydrocarbon with HNO3 and H2SO4, followed by splitting off H2SO4 and H2O.
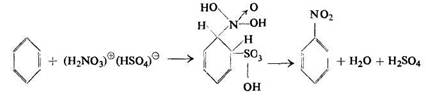
Usanovich also assumed Hantzsch's cations to be the nitrating agents in a mixture of nitric and sulphuric acids. He believed that in the nitration process the nitracidium cation was attached first accompanied by splitting off water:
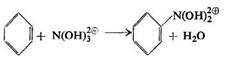
On dilution with water the resulting new cation, C6H5N(OH)22+, gives nitrobenzene:
C6H5NO2H22+ + 2H2O => C6H5NO2 + 2 H3O+
In the nitration of aliphatic hydrocarbons the NO3 ion reacts:
R CH3 + NO3- <=> R CH2NO2(OH)-
The anion formed may undergo a hydrolysis process in an acid medium:
R CH2NO2(OH)- + H3O+ <=> R CH2NO2 + 2H2O
In favour of the view, that postulates the formation of an addition product during the first stage of nitration this fact should be known to all who are practically engaged in nitration of aromatic hydrocarbons. Immediately before contacting the nitrating acid (HNO3 or nitric and sulphuric acids mixture), benzene and toluene give brown coloured products amid nitric acid vapours. On dissolution in the acid these products decolourize at once. It is quite possible they arc addition products formed by nitric acid vapours with the hydrocarbon.
The existence of similar addition products must be mentioned here. Steinkopf and Kuhnel observed that benzene reacted with nitryl chloride at room temperature under pressure to yield l-chloro-2-nitrocyclohexadiene, which on heating released a molecule of hydrogen chloride giving nitrobenzene:
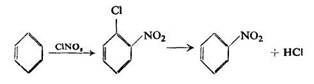
Thus, there is evidence that such addition is probable and it seems to confirm the basic scheme of Thiele-Holleman-Wieland, assuming that under certain conditions substitution with the NO2 group may be preceded by the formation of addition products.
Although this view was replaced by the conception of the nitration reaction as double exchange reaction, it seems that the mechanism of the nitration reaction is rather a complicated one and under various conditions may proceed differently. The mechanism which includes addition may also exist, especially at low temperatures, and may not necessary proceed under the influence of the nitronium ion. It seems that nitric acid in the form of HO-NO2 can be the nitrating agent acting through the addition mechanism.
Studies of the nitration of terpenes are of interest too, as they provide evidence for the possibility of attaching a HNO3 molecule to the double bond. Konovalov obtained nitro derivatives from menthene, camphene, pinene and bornylene on acting with nitric acid. Bouveault was able to prepare an addition product of camphene and HNO3. He obtained an oily product with a structure that could not be well defined. The reaction of addition of nitric acid to the double bond was studied in detail by Sucharda. He found that on acting on pinene with nitric acid containing 33% of KNO3 instead of with pure nitric acid, or by introducing nitric acid vapours diluted with dry air, nitric acid esters were obtained in over 70% yield. When reduced with zinc dust in the presence of ammonia, the esters were converted to the corresponding alcohols.
Using both methods Sucharda obtained: borneol (I), fenchol (II) and terpineol (III) nitrates :
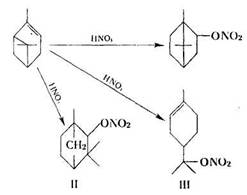
H. Kuczynski and L. Kuczyliski extended Sucharda's observations in their studies on other terpene hydrocarbons. They obtained isoborneol nitrate (IV) on reacting camphene with concentrated nitric acid (without KNO3):
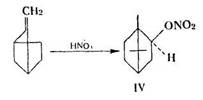
From bomylene they obtained isobomeol (V) and epibomeol (VI) nitrates:
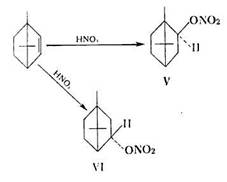
H. Kuczynski and L. Kuczynski have also studied the action of nitric acid on p- and 5- fenchene, limonene, sylvestrene and other terpene hydrocarbons.
It has also been shown that the addition of nitric acid molecules to olefins is not the only possible reaction of olefins with HNO3. Formation of nitro-olefins, i.e. ordinary nitration by substitution, is also likely.
| ДонНТУ | Портал магистров | Биография | Автореферат | Библиотека | Ссылки | Отчет о поиске |