DonNTU > Master's portal>Curriculum vitae | Links | Report about the search | Library
SUMMARY
RUS
Donetsk national technical university
Bida Natalia
Speciality: "Chemical technology of high molecular connections"
THEME OF MASTER'S WORK:
" RESEARCH OF PROTECTIVE WATER-CONTAINING EXPLOSIVE MATERIAL PROPERTIES "
The new type of industrial explosive materials is water-filled explosive materials (WEM), which posses essential advantages in comparison with explosive material like powder. WEM differ with their high efficiency, relatively maximum safety and ecologic cleaning, possibility to regulate its detonative characteristics, stability of structure and water-resistance.
To rise power characteristics of WEM, the water in their structure is substituted for solution of saltpeter, called mother liquor.
The aim of this work is to find maximum solubility for mixture of saltpeter and to determine influence of mother liquor on the fullness of detonation.
While carrying out the work data about solubility of saltpeters are obtained mixtures: calcium saltpeter (Ca(NO3)2), ammonium saltpeter (NH4NO3) and sodium saltpeter (NaNO3 ), both chemically clean and technical saltpeters.
For investigation of mutual solubility the experiments how to determine solubility of one component in saturate solution of other have been carried. For this 100 ml of water has been saturated with one component. Into this solution the material was introduced with small doses and it's solubility was determined with the help of titration method (table 1).
Table 1 – Solubility of salts
Salt |
Qualification |
Solubility of salts g/on 100 g H2O in t°C |
-5 |
0 |
+5 |
+10 |
+15 |
+20 |
+25 |
+30 |
+35 |
NaNO3 |
ccl |
72,34 |
73,00 |
76,3 |
80,51 |
83,5 |
88,0 |
92,7 |
96,5 |
- |
NaNO3 |
tech |
48,0 |
57,0 |
66,56 |
72,6 |
77,0 |
81,6 |
87,5 |
94,57 |
99,4 |
Ca(NO3)2 |
ccl |
87,5 |
95,5 |
100 |
115 |
122 |
129 |
138 |
150 |
170 |
Ca(NO3)2 |
tech |
- |
- |
83,5 |
106 |
110 |
118 |
125 |
136 |
142 |
NH4NO3 |
ccl |
115 |
118 |
- |
144,5 |
167,4 |
177 |
214,4 |
242 |
- |
NH4NO3 |
tech |
- |
112 |
- |
133 |
- |
172 |
- |
210 |
- |
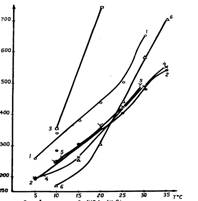
According to results of experiment graphic of solubility depending on temperature was made (fig.1,2,3). For comparison solubilities of respective materials in 100 ml of water are given on these graphics.
From fig.1 we can see, that solubility of calcium saltpeter is growing up drastically in presence of ammonium saltpeter. The similar picture is observed at dissolution of ammonium saltpeter in calcium saltpeter solution (fig.2). Thus, it can be expected that if we add these components into explosive material the consistency changes remarcably towards the ”dilution” of composition.
Remarcable influence on solubility of calcium saltpeter is shown by sodium nitrate (fig.1). Though under the middle temperatures (20-30 °C) of the chosen interval the solubility of calcium saltpeter decreases insignificanty, if there is sodium nitrate for more low temperatures (10-17 °C) this difference become considerable. Fully probably that in condition of low temperatures the action of sodium nitrate can be expressed owing to cristallization of calcium saltpeter.
  |
  |
  |
Fig.1-Solubility of Ca(NO3)2
1-chem. cl.;
2-tech;
3-in n.m. NH4NO3;
4-in n. m. NaCl;
5-in 25% m.CO(NH2)2;
6-in n. m. NaNO3.
|
Fig.2-Solubility of NH4NO3
1-chem. cl.;
2-tech;.
3- in 25% m.CO(NH2)2;
4-in n. m. Ca(NO3)2•4H2O.
|
Fig.3-Solubility of NaNO3
1-chem. cl.;
2-tech.;
3- in n. m. Ca(NO3)2•4H2O ;
4- in n. m. NaCl.
|
Least of all the influence of carbamide supplement is expressed. It rises the solubility of technical calcium saltpeter a bit and it doesn’t show a significant action on the solubility of pure calcium nitrate (fig.1). However, solubility of ammonium saltpeter in presence of calcium saltpeter grows greatly. Reciprocal dissolution of calcium and chlorine nitrates practically equal to zero.
Thus, the investigations, which have been carried out, allow us to find some moments of reciprocal influence of components, their compatability in explosive water-filled compositions and to approach more substantially to composing of compounds.
DonNTU > Master's portal>Curriculum vitae | Links | Report about the search | Library