An Objective Characterization of Atherosclerotic Lesion
An Alternative Method to Identify Unstable Plaque
Sayed Aly, PhD, FRCS; Christopher C. Bishop, MCh, FRCS
Stroke 2000;31;1921-1924
Stroke is published by the American Heart Association. 7272 Greenville Avenue, Dallas, TX 72514
Copyright © 2000 American Heart Association. All rights reserved. Print ISSN: 0039-2499. Online ISSN: 1524-4628
Background and Purpose—The aim of this study was to evaluate a computer-assisted technique to characterize atherosclerotic plaque.
Methods—In 9 subjects (7 men, 2 women; mean age 33 years), known anatomic areas (carotid, fat, muscle, iliotibial tract, and tibia) were scanned with an Acuson duplex ultrasound machine with 72 machine settings. The ultrasound images of these anatomic areas were recorded on magneto-optical disks. Echo amplitude statistics were obtained, and the mean pixel value (MPV) was used to assess the level of echogenicity. The ideal settings of this particular scanner for optimal discrimination between these tissues types were identified by the Heuristic Index of Discrimination. With these settings, carotid artery scanning was performed on 17 patients (15 men, 2 women; mean age 65 years), and the image analysis of their ultrasound carotid plaques was compared with their histological findings.
Results—In this study, discrimination between the selected tissues was found to be optimal when the controls were set at log 40 dB, 0/2/0, and gain of 25 dB. The MPV of the carotid specimens scanned at this setting correlated significantly with the histological findings (Spearman correlation, P=0.002).
Conclusions—Computer-assisted image analysis to give the MPV is a technique that may be used to identify unstable atherosclerotic plaques reliably. (Stroke. 2000;31:1921-1924.)
Key Words: atherosclerosis, image processing, computer assisted
The use of B-mode pulsed echo imaging to identify the vessel wall was first introduced by Olinger [1]. Plaque structure has been demonstrated as an important factor in the development of embolic events[2]; therefore, characterization of carotid plaque in asymptomatic patients may allow the prediction of risk of a subsequent cerebral event.
Despite the advances in ultrasound imaging in many applications, plaque characterization has remained a subjective process that is observer dependent. Consequently, the value of the Gray-Weale classification[3] has been questioned because it is observer dependent and subject to errors that may arise from different settings of the ultrasound scanner, and it could be improved by application of modern image processing.
It has been demonstrated [4] that digital image processing enables the maximum information from such images to be obtained. Every image consists of a range of shades of color and intensity. In digital images, numbers are used to express the displayed brightness. Each pixel is typically stored as an 8-bit byte giving a representation of 1 of 256 shades of gray; the brightness is represented by the value 0 for black and 255 for the brightest white spot.[5–8]
In computer-assisted image analysis, many statistical processes can be used (see Figure 1). Use of computerized image processing has been described previously in radiological diagnosis[9– 13] to evaluate ultrasound images and correlate statistics with various pathological conditions.
In the present study, image statistics have been used to evaluate plaque morphology[4]. The mean pixel value (MPV) of the echoes from the plaque was used as an index of echogenicity, and its value as a measure to characterize atherosclerotic plaque was assessed.
Subjects and Methods
Instrumentation
An Acuson duplex machine 128 XP/10 duplex scanner (Acuson 1220) was used. An Apple Macintosh computer was connected to the Acuson ultrasound machine. The Adobe Photoshop 3.0.4 program (Adobe System Inc) was used. The area of interest was selected and the image analyzed. MPV and a histogram were obtained (see Figure 1). The specimen tank for ultrasound imaging of excised atheromatous plaques was made of acrylic sheets in which the carotid plaque could be mounted and scanned. Finally, a personal computer was connected to a CCD camera (Microscope-Nikon Labophot-2 camera, JVC TK-1281) installed on a microscope (Olympus) and supported with a Lucia image-analysis computer program (version 3.52a, laboratory imaging for Nikon UK).
Clinical Studies
There are a number of user-adjustable variables on ultrasound machines that affect the image. The exact nature of the processing involved varies from machine to machine. The settings on the Acuson machine were investigated to determine which gave the greatest differentiation between a selection of tissues, so that appropriate standardized settings could be used to ensure that all data from the in vitro study were consistent. Optimum values were estimated by a Heuristic Index of Discrimination (HID; see Statistics). The controls in the Table were optimized.
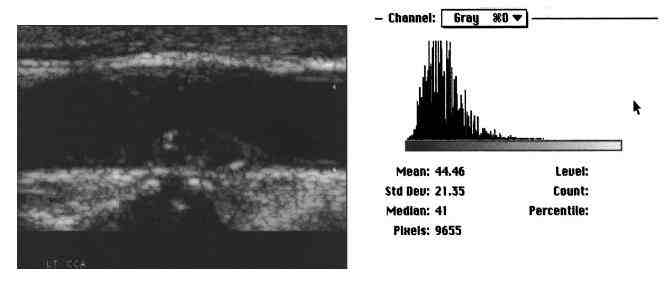
Figure 1. Image analysis of carotid plaque. This figure demonstrates atherosclerotic carotid plaque and the image histogram and statistics.
Nine healthy subjects (7 men and 2 women; mean age 33 years) who had no history of connective tissue disease and were receiving no treatment for any condition were scanned with the Acuson with a 7-MHz linear array transducer. The following anatomic areas were examined: carotid artery (for assessment of blood), subcutaneous fat (axillary and abdominal), muscle (quadriceps and abdominal), iliotibial tract (for assessment of fibrous tissue), and tibia and skull (for assessment of calcified structures). These structures were scanned while the controls were adjusted and images were acquired for analysis. Each of the anatomic regions was scanned, and the image was recorded at 72 different settings of the ultrasound machine, obtained by adjusting each of the above parameters one at a time. All images were electronically recorded on magneto-optic disks (128 MB). The Adobe Photoshop computer program was used to examine the stored image, the area of interest was selected with the program’s selecting tool, and the MPV of the selected anatomic region was obtained. The optimum setting of the scanner was defined as the setting at which the MPV was best able to discriminate between tissues.
Further study was undertaken to verify the relation between the MPV and the atherosclerotic plaque structure by use of the optimum machine setting. Patients with significant carotid artery stenosis who were scheduled to undergo carotid endarterectomy were scanned before the surgery. Carotid plaques were classified according to Gray-Weale classification. Highly calcified plaques were excluded on the basis of scanning to avoid the effect of acoustic shadowing on image analysis. After endarterectomy was performed, the excised specimens were examined and scanned within 4 hours. All plaques were scanned in both longitudinal and transverse sections with the equipment adjusted at the optimum control settings. The examination started from the cranial end of the plaque and proceeded toward its caudal end. The probe was moved along at 0.5-cm intervals, and transverse images of the plaques were acquired by the computer system and recorded.
The area of interest on the stored images was highlighted to obtain the MPV of the plaque. Each specimen was fixed in formalin-saline 10% for 24 hours. All specimens were anatomically oriented and embedded in paraffin, with the common carotid end at the base and the internal carotid end at the top. Specimens were cut transversely at 0.5-mm intervals along the whole specimen length and stained with elastin van Giessen. Slides were examined by the histology image-analysis system to assess the percentage area of soft materials (fat and blood) and fibrocalcific plaque. The area of histological section occupied by each tissue type was calculated with the aid of the Lucia computer program. To validate the computer-assisted histology image analysis, and to measure the agreement between observers to identify different histological structures, 10% of the carotid histology specimens were reexamined by one of the authors within 6-month intervals, as well as by another independent researcher. Both the intraobserver and interobserver variations were obtained by the Bland and Altman technique[14].

