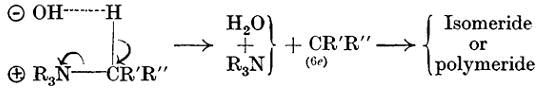
The explanation given in Part V (preceding paper) of the different modes of thermal decomposition of quaternary ammonium hydroxides requires that, in suitable circumstances, olefin-elimination should replace paraffin-elimination in the degradation of phosphonium hydroxides, but that, on the contrary, paraffin-elimination should never, even under the most favourable structural conditiom, replace olefin-elimination in the thermal decomposition of ammonium hydroxides. The first of these inferences has already been verified; the second led to the experiments now described.
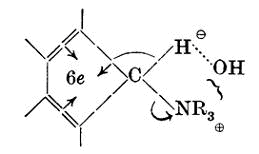
Having in mind the principle that the facility of the paraffinic degradation depends essentially on the anionic stability of the group eliminated (Part V), we could think of no structural condition more favourable to the observation of paraffinic thermal decomposition in the ammonium hydroxide series than that which is attained by the attachment of a 9-fluoryl or similar group to the quaternary nitrogen atom. In fluorene and its analogues the dominating circum-stance is that the powerful forces of aromatic stability, acting as explained by Goss and Ingold (J., 1928, 1268), are working for the separation of a 9-fluoryl anion. Subsidiary factors also collaborate, and one illustration of the combined effect is that fluorene is unique amongst the simpler hydrocarbons in yielding a potassium salt by direct action on potassium hydroxide.
We prepared salts of the fluoryl-9-trimethylammonium and -9-triethylammonium series and studied the decomposition of the corresponding hydroxides. In neither case was any trace either of fluorene or of an amine oxide produced, and the inference regarding the incapacity of ammonium hydroxides for paraffinic thermal decomposition is thus confirmed to that extent.
Fluoryl-9-trimethylammonium hydroxide gave a small amount of dimethyl-9-fluorylmine and a high yield of trimethylamine; part of the corresponding quantity of 9-hydroxyfluorene appeared as 9-fluoryl ether, and this is in accordance with Barbier’s observation of the production of this ether by the action of heat on the hydroxycompound (Ber., 1575, 8, 829; Ann. Chim. Phys., 1876, 7, 507). The most interesting observation, however, was that a considerable proportion of the material which might have appeared as 9-hydroxyfluorene or its ether was actually recovered as bis-00’-diphenylyleneethylene (difluorylidene), and, as this is not formed from either the hydroxy-compound or the ether under conditions equivalent to those of the experiment, it is evidently a primary product of the decomposition. Fluoryl-9-triethylammonium hydroxide gave no perceptible quantity of diethyl-9-fluorylamine and no ethylem; otherwise it behaved similarly to the trimethyl compound.
These appear to be the first examples of 1:1-elimination from a quaternary ammonium hydroxide. Hanhart and Ingold pointed out (J., 1927, 1000) that despite the greater inductive effect of the electron-attracting ammonium pole on the α- than on the β-hydrogen atoms, the decomposition of ammonium compounds does not normally involve attack by the anion on the incipiently ionised α-hydrogen atom, because, whilst in β-proton-attack the octets are preserved throughout, α-proton-attack necessitates the degradation of an octet to it sextet:
9-Bromofluorene.-9-Hydroxyfluorene (15 g.), prepared from fluorenone as described by Werner and Grob (Ber., 1904, 37, 2895), was dissolved in the minimal amount of glacial acetic acid at 30–35o and the solution, mixed with an equal volume of a solution of hydrogen bromide in glacial acetic acid (600 g./l.), was kept at room temperature over-night (crystals separated). The 9-bromofluorene (20 g., m.p. 103–104.5o ), which was collected after addition to the mixture of an equal volume of water, required no purification (compare Staudinger, Ber., 1906, 39, 3061).
Fluoryl-9 -trimethylammonium Salts. –Bromofluorene (6.1 g.) was shaken with ethyl-alcoholic trimethylamine (20 c.c. ; 330 g./l.) until the solid had dissolved and heat ceased to be evolved. The mixture was then heated at 50o for 1 hour and the fluoryl-9-trimethylammonium bromide, m.p. 189–190o (decomp.), was precipitated by addition of ether, collected, and washed with ether (Found: Br, 24.8, 24.8. C16H18NBr requires Br, 26.3%). The picrate, precipitated by addition of the bromide to aqueous sodium picrate, separated from alcohol in plates, which softened at 170o and melted at 175o (Found: C, 58.0; H, 4.6. C22H20O7N4 requires C, 58.3; H, 4.6%).
Decmposition of Fluoryl-9-trimethylammonium Hydroxide. – The hydroxide solution, prepared from the bromide and carbonate-free silver oxide, had a strong purple colour (the tentative suggestion may be made that this is due to partial conversion of the hydroxide into the presumed precursor of C12H8 >C:C< C12H8), which disappeared soon after the commencement of concentration; rapid decomposition then set in, which was completed in a metal-bath at 170o . A red-brown resinous residue remained, and that portion of the volatile basic products which did not condense with the distillate was trapped in hydrochloric acid; no gas passed through the acid traps during the progress of the decomposition, which was followed by periodically titrating the distillate, and when reaction had ceased the apparatus was swept out with nitrogen. Methyl alcohol could not with certainty be detected by distilling the neutralised distillate and treating the first runnings with p-nitrobenzoyl chloride and pyridine. Formaldehyde, which would be formed from trimethylamine oxide at the temperature of the experiment, was also tested for with negative results. The volatile bases recovered (94%) from the distillate and contents of the acid traps consisted essentially of trimethylamine (hydrochloride and picrate prepared), but contained small amounts of impurities, including traces of a base sparingly soluble in water (possibly dimethyl-9-fluorylamine). The resinous residue was extracted first with boiling water, from which a scarcely ponderable residue was obtained on evaporation, and then with hot 5% hydrochloric acid. The acid extract on basification (small precipitate) and extraction with ether yielded a small amount (<1%) of a solid amine, which was purified by distillation and identified as dimethyl-9-fluorylamine by comparison with a specimen prepared as described below (long needles, m.p. and mixed m.p. 49–50o ). The identification was further confirmed by treating both specimens of the tertiary amine with 2,4,6-trinitroanisole in benzene, thus converting them into fluoryl-9-trimethylammonium picrate.
The resin remaining after the extractions with water and acid was dried in benzene solution and divided into three portions. One was used to show that 1% of added fluorene could be detected by sublimation, and thereafter for other exploratory experiments. The second was tested for fluorene by sublimation, with negative results, and was then treated with an equal volume of benzene and an excess of picric acid. The picrate which, together with excess of picric acid, separated from the boiled and ooled solution was separated from the viscous mother-liquor by draining on porous porcelain, and a portion was crystallised twice from benzene, from which red-brown needles separated, which were identified as bis-oo'-diphenylylene-ethylene picrate by direct comparison with an authentic specimen. The remainder of the crude picrate was digested with warm aqueous ammonia and the bis-oo'-diphenylylene-ethylene was collected and crystallised first from glacial acetic acid and then from chloroform-alcohol. It was identified by direct comparison (vermilion needles, m.p. and mixed m.p. 189–190o ), by analysis (Found: C, 94.9; H, 4.9. Calc.: C, 95.1; H, 4.9%), and by conversion into its dibromide, which was prepared in chloroform solution, precipitated with ligroin, and crystallised from chloroform-ligroin (nearly colourless prisms, m.p. and mixed m.p. 236o , decomp.). The remainder of the resin was separated by crystallisation from acetic anhydride into an amorphous fraction, which was not further examined, a red crystalline fraction consisting largely of bisdiphenylylene-ethylene, which was isolated from it through the picrate, and a yellow crystalline fraction, m.p. 165–180o , consisting largely of 9-fluoryl ether, coloured by the hydrocarbon and some other impurity (see later). Satisfactory purification of the ether could not be effected, and the crude product in benzene-acetic acid solution was therefore treated with hydrogen bromide at 40o for 16 hours in order to convert the ether into 9-bromofluorene. Water was added, and the benzene layer removed, washed with water, dried, and mixed with alcoholic trimethylamine. After being kept over-night, the mixture was evaporated to dryness, re-evaporated after addition of water, and extracted with warm water. The extract on treatment with saturated aqueous sodium picrate yielded fluoryl-9-trimethylammonium picrate. The residue insoluble in water was semcrystalline, and crystallisation from acetic acid gave a small amount of an unidentified orange substance, m.p. 280o (decomp.). The bisdiphenylylene-ethylene required for comparison was prepared by Graebe and Stindt's method (Annalen, 1896, 291, 2).
Dimethyl-9-fluorylamine was obtained by warming 9-bromofluorene with excess of 20% aqueous-alcoholic dimethylamine at 70–80o for 3 hours, and pouring the product into water. The base, which was extremely soluble in anhydrous solvents, and tended to separate as an oil from aqueous alcohol, was most easily purified by distillation as stated above (Found: C, 86.0; H, 7.3. C15H15N requires C, 86.1: H, 7.2%). Its picrate crystallised from alcohol in leaflets, m.p. 203–204o (Found: C, 57.5; H, 4.3. C21H18O7N4, requires C, 57.3; H, 4.1%).
Fluoryl-9-triethylanammonium Salts.–Bromofluorene (6.1g.), triethylamine (6 c.c.), and alcohol (15 c.c.) were heated together under reflux on the steam-bath for 3 hours. The quaternary ammonium bromide wag precipitated by addition of ether, redissolved in alcohol, and reprecipitated with ether. The picrate, prepared from the bromide and aqueous sodium picrate, crystallised from alcohol in plates, m.p. 166o (Found: C, 60.5; H, 5.2. C25H26O7N4 requires C, 60.7; H, 5.3%).
Decmposition of Fluoryl-9-triethylammonium Hydroxide.–The purple solution of the hydroxide was distilled as in the preceding case, with parallel results except that diethyl-9-fluorylamine was not obtained. Triethylamine picrate had m.p. 175o (Jerusalem, J., 1909, 95, 1281, records 173o ). Bis-oo'-diphenylylene-ethylene (Found: C, 94.7; H, 5.0%) had m.p. and mixed m.p. 189–190o , i.e., 194–195o corr. (Graebe and Maritz, Annalen, 1896, 290, 241, record 187–188o corr.).