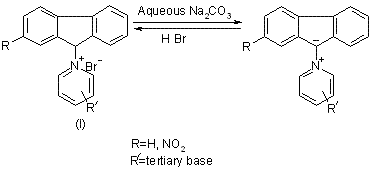
J. P. Saxena and R. Kulshreshtha
Decomposition of 9-Ammonium and 2-Nitro-9-Ammonium Fluorenylides in Solution.
Jour. Indian Chem. Soc., Vol. 48, ¹2, 1971
The decomposition of 9-arnmonium and 2-nitro-9-ammonium fluorenylides is found to take place through the oxidative cleavage of polar carbon nitrogen bond. During this process the fluoryl radical moiety of the ylid gets oxidised to the corresponding fluorenone and the tertiary base is obtained back.
Ylids have been classified under the head of > ĉ- betaines [1] possessing a carbon anion and a cationic function within the molecule. 9-Ammonium fluorenylides are– the ylids which are derived from the quaternary 9-ammonium fluorenyl halides (I) by treating their aqueous solution with silver oxide, silver carbonate or preferably 2N sodium carbonate solution. Their mode of derivation involves the loss of a molecule of water from the onium-hydroxide and as such they may also be classified as the anhydro-salts [2,3]:
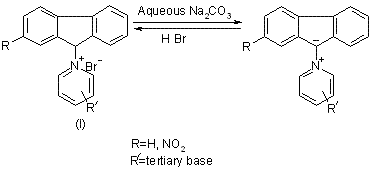
Fluorenylides are readily extracted into chloroform to which they impart a deep blue colour. Their deep blue colour, the dipolar nature and the property of solvent interaction is comparable with the azulenes [4]. The deep colour of these fluorenylides starts fading as soon as they are generated in chloroform. Some of the ylids containing, nitrogen atom situated in an aliphatic tertiary amine residue, are found to be of fleeting existence in solution which is exposed to air.
Fluorenylides are readily extracted into chloroform to which they impart a deep blue colour. Their deep blue colour, the dipolar nature and the property of solvent interaction is comparable with the azulenes [4]. The deep colour of these fluorenylides starts fading as soon as they are generated in chloroform. Some of the ylids containing, nitrogen atom situated in an aliphatic tertiary amine residue, are found to be of fleeting existence in solution which is exposed to air.
EXPERIMENTAL
9-Ammonium and 2-nitro-9-ammoniium fluorenyl bromides: These were obtained by boiling a solution of 9-bromo fluorene or 2-nitro-9-bromo fluorene with a slight excess of the heterocyclic tertiary base in Analar acetone under reflux on a water bath. While refluxing the solution a bright red compound separated. The yield of the red compound increased with the increase in the time of reflux. The red colour and its high melting point showed that it was the bis-phenylene derivative of fluorene [7]. In order to decrease the formation of this red compound a suitable optimum time had to be adjusted, which was found to be sufficient for the formation of the corresponding fluorenyl bromide. The quaternary salt was separated from the red compound by dissolving it in ethanol. It was then recrystallised from ethanol-ether mixture. Kröhnke prepared pyridinium; quinolinium and isoquinolinium fluorenyl bromides by keeping the respective base and 9-bromo fluorene in a suitable solvent for a few days (2 months in the case of quinoline). Table 1 gives an account of the various quaternary fluorenyl bromides which have been prepared.
Most of these quaternary ammonium fluorenyl bromides were highly hygroscopic and they had to be thoroughly dried and then preserved in a vacuum desiccator. All the m.pts. have been determined on a Kofler instrument and are uncorrected.
9-Ammonium and 2-nitro-9-ammonium fluorenylides: The fluorenylides were generated from the corresponding quaternary fluorenyl bromides by the treatment of their aqueous solution in a limited quantity of water with 2N sodium carbonate solution. The deep blue colour of the ylid was extracted into chloroform. This process was repeated a couple of times till no more colour was developed in the chloroform layer. The chloroform layer was dried over anhydrous sodium carbonate in dark, and then filtered. It was concentrated by evaporation under vacuum and then light petroleum was added. The 9-ammonium fluorenylides could not be obtained in solid state owing to their rapid composition. Some of the 2-nitro-9-ammonium fluorenylides could be obtained as dark amorphous powder from the chloroform petroleum ether mixture. A trace of this powder imparted deep blue colour of the ylid to the chloroform layer. The identity of these ylids was established by the fact that the m.p. of their picrate was the same as the picrate of the corresponding quaternary fluorenyl bromide from which they were generated, and that the mixed m.p. of their picrates remained undepressed.
Decomposition of fluorenylides: The deep blue colour of the solution of the fluoren ylides in chloroform or benzene turned yellow on keeping. The reaction is found to be highly sensitive to light and it commences without any period of induction. It was observed that the blue colour of the ylid persisted for a longer period, when the chloroform used for their extraction was freed from any traces of dissolved oxygen by bubbling nitrogen through it and then preserving the solution in a tightly stoppered flask in dark. The flasks were kept filled upto the neck for the rigid exclusion of air. A rapid fading of the colour of the ylid took place when ordinary chloroform was used and the solution was kept exposed to air. The fading of colour took place slowly in an inert atmosphere. This shows that the decomposition of ylids is caused by the presence of atmospheric oxygen which is responsible for their autoxidation. The products of oxidation of fluorenylides were separated and identifiedas follows.
A blue solution of the desired fluorenylide in chloroform was exposed to air in diffuses light. The blue colour started fading and after some time the colour of the solution turned yellow. This yellow solution was evaporated and the residue was taken up in benzene. It was chromatographed over alumina using benzene as eluent. The faster moving band contained negligible amount of the solid material but the second bright yellow band gave a yellow solid which was crystallised from ethanol. Its m.p. and mixed m.p. determination showed that it was fluorenone or 2-nitro fluorenone, according to the type of fluorenylide taken. The third band eluted with ethanol-benzene mixture yielded a viscous residue which smelt of the tertiary base that formed a part of the fluorenylide skeleton. These bases were identified through their picrates. The subsequent bands on washing down the column with ethanol yielded resinous mass which could not be identified. The results obtained are given in table
Table 1 – DECOMPOSITION OF 9-AMMONIUM AND 2-NITRO-9-AMMONIUM FLUORENYLIDES
| Name of compound | Time of reflux | M.p.,oC | Yield, % | Analysis, % | M.p. of the derivative, oC |
| 9-pyridinium fluorenyl bromide C18H14NBr | 1 hr | 194 | 36 | Found N, 4.3; Br 24.4. Requires N, 4.3; Br. 24.6. |
Picrate 142 |
| 9-α-picolinium fluorenyl bromide C19H15NBr | 15 min | 196-97 | 35 | Found N, 4.2; Br 23.5. Requires N, 4.14; Br. 23.6. |
Perchlorate 148-51 |
| 9-β-picolinium fluorenyl bromide C19H15 |
15 min | 162 | 25 | Found N, 4.0; Br 23.2. Requires N, 4.14; Br. 23.6. |
Perchlorate 124 |
| 9-γ-picolinium fluorenyl bromide C19H15NBr | 15 min | 173-74 | 30 | Found N, 4.1; Br 23.0. Requires N, 4.14; Br. 23.6. |
Perchlorate 119 |
| 9-isoquinolinium fluorenyl bromide C22H16NBr | 1 hr | 234 | 40 | Found N, 3.7; Br 21.0. Requires N, 3.74; Br. 21.38. |
Picrate 190 |
| 9-quinolinium fluorenyl bromide C22H16NBr | 4 hr | 217-18 | 20 | Found N, 3.69; Br 21.2. Requires N, 3.74; Br. 21.38. |
Perchlorate 181 |
| 2-nitro-9-pyridinium fluorenyl bromide C18H13N2O2Br | 3 hr | 126-28 | 40 | Found N, 7.2; Br 21.4. Requires N, 7.6; Br. 21.6. |
Picrate 228 |
| 2-nitro-9-α-picolinium fluorenyl bromide C19H15N2O2Br | 1 hr | 121 | 40 | Found N, 6.9; Br 20.6. Requires N, 7.31; Br. 20.88. |
Picrate 153 |
| 2-nitro-9-β-picolinium fluorenyl bromide C19H15N2O2Br | 1 hr | 112 | 35 | Found N, 7.01; Br 20.2. Requires N, 7.31; Br. 20.88. |
Picrate 140 |
| 2-nitro-9-γ-picolinium fluorenyl bromide C19H15N2O2Br | 1 hr | 133 | 40 | Found N, 6.89; Br 20.4. Requires N, 7.31; Br. 20.88. |
Picrate 161 |
| 2-nitro-9-isoquinolinium fluorenyl bromide C22H15N2O2Br | 2 hr | 230 | 35 | Found N, 6.3; Br 18.8. Requires N, 6.68; Br. 19.06. |
Perchlorate 200 | 2-nitro-9-quinolinium fluorenyl bromide C22H15N2O2Br | 2 hr | 204 | 20 | Found N, 6.35; Br 18.6. Requires N, 6.68; Br. 19.06. |
Perchlorate 231 |
Table 2
| Name of the m-fluorenylide | Shape and yield | M.p., oC | M.p. of picrate, oC | M.p. of picrate of the base obtained on decomposition, oC |
| 2-nitro-9-pyridinium fluorenylide | Black green amorphous powder. 60% | 200 | 227–30 | 164 |
| 2-nitro-9-α-picolinium fluorenylide | Dark powder. 55% | Does not melt | 153 | 168 |
| 2-nitro-9-β-picolinium fluorenylide | Black powder | Decomposes | 140 | 152 |
| 2-nitro-9-γ-picolinium fluorenylide | Dark amorphous powder | – | 161 (with decomposition) | 166 |
| 2-nitro-9-isoquinolinium fluorenylide | Unstable. Not isolated | – | – | 221 |
| 2-nitro-9-quinolinium fluorenylide | Unstable. Not isolated | – | – | 202 |
Compounds 1 to 6 gave 2-nitro fluorenone on deocomposition. Similarly the 9-ammonium fluorenylides which were isolated only in solution, gave fluorenone on decomposition, m.p. 84°; 2,4-DNP m.p. 284°; together with the heterocyclic tertiary base which was identified as above.
The authors are grateful to Prof. R. C. Kapoor for providing research facilities. One of us (R.K.) is grateful to the Ministry of Education for the award of a research scholarship.