Research of synthesis diazepinov as adaptive-genes of negative ecological influence
Leader of work:Bulavin A.V.
Materials on the theme of master's work:
Abstract | Links | Report about the searchProtection of surrounding environment at conditions of rough growth of industrial production became one of the most actual problems of modernity. Polluted natural environment can negatively influence for "recipients" (people, industrial, transport and housing-and-municipal objects, agricultural lands, forests, water reservoirs and etc), that can be result in increasing of people grouth deseases. In connection with this one of the main tasks is working out of new medical preparations, which usage would lead to the lowering of percentage of population, suffered from different kind of deseases.
At materials of the last conference we informed about usage of hetero-cyclic systems with entering of the hydrazine at organic synthesis, and namely for obtaining of diazepines, which in most cases are structural components of alkaloids.
This work is devoted to obtaining of new seven-member hetero-cycle, possessing pharmacological action, and whose analysis allows to observe dependence of influence of quantity of metoxigroups at hetero-cyclic systems for efficiency of such action.
[4-(3,4-dimetoxiphenil)-2,5-dihydrobenzole[d]-2,3-diazepine-1-iliden]-hydrazine is an analogue of the compound, which can serve the base of synthesis of new medical preparations and biologically active substance [1]. It is designed by ghemistry department of biologically active compounds of InFOU named after L.М.Litvinenko NAS of Ukraine. The process of obtaining of such cycle is rather complicated and consists of four stages. Consequence of proceeding reactions is schematically represented at figure 1.
On the first stage crystal homophtale acid (1) is reacted with verotrol (2). For acceleration of reaction proceeding to obtained solution we add catalyst. In this case as catalyst poly-phosphorous acid is. Reaction mixture is warmed on water bath within one and a half hour. As result образуется the intermediate product is formed 4-(3,4-dimetoxiphenil)-isochrome1-он (3). Its extraction is not large and it was 33%. Melting temperature for crystals is 155-157 °С.
The second stage is interaction of obtained on the first stage isochrome-[1]-оn and hydrazine. In this case not pure hydrazine is used, but its monohydrate. Initial components are loaded into flask, to which the reversed refrigerator is connected. Hushing of reaction mixture is one of the optimal conditions of process conducting. Therefore the flask is equipped with mixture device. The solvent at the second stage is isopropanol. Mixture is warmed up to boiling and within one and a half of an hour we continue to boil up to appearing of white crystals - 4-(3,4-dimetoxiphenil) - diazepine-1-оn (4). Then these crystals are filtered at Byukhner funnel and dried drying shelf. Extraction 4-(3,4-dimetoxiphenil)-diazepin-1-оn (4) was 90%. Melting temperature for crystals became 174-176 °С.
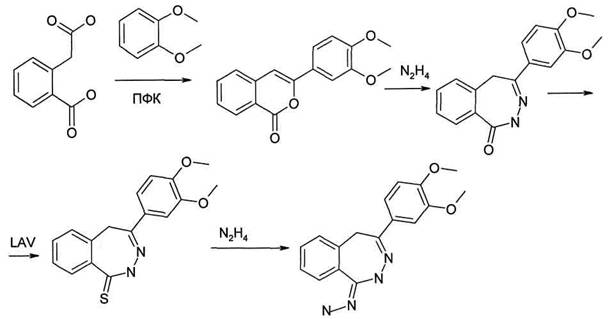
Figure 1 – Consequence of stages of synthesis [4-(3,4-dimetoxiphenil)-2,5-dihydrobenzole[d]-2,3-diazepine-1-iliden]-hydrazine
The next stage is obtaining 4-(3,4-dimetoxiphenil)-diazepine-1-tion (5). We place into the flask 4-(3,4-dimetoxiphenil)-diazapine-1-оn and solvent, which is ethyl spirit. We add the reactant of Lavesson at the moment of boiling of solution, after that we switch on reverse refrigerator and warm it within two hours.
For preparation of Lavesson reactant at the flask, equipped with reverse refrigerator, we mix absolute anisol sulfide of phosphorus. The solution is being boiled within five hours. Characteristic attribute of reaction proceeding is extracting of hydrogen sulfide gas. Then the flask is cooled and large crystal sediment is filtered, and washed out by pure anisol. Sediment on filter is not dried by us, but we place it at once into hermetical utensil.
The final stage of obtaining [4-(3,4-dimetoxiphenil)-2,5-dihydrobenzol[d]-2,3-diazepine-1-iliden]-hydrazine is reaction of interaction of diazepine - 1 -tion with hydrazine. This reaction takes place at the flask, equipped with reverse refrigerator, within 30-40 minutes from moment of boiling of solution.
Thus, synthesis [4-(3,4-dimetoxiphenil)-2,5-dihydrobenzol[d]-2,3-diazepine-1-iliden]-hydrazine will give the opportunity to detect some dependences of influence of structure of hetero-cyclic systems with entering of hydrazine for efficiency of pharmacological action.
ДонНТУ Портал магистров ДонНТУ Реферат | Библиотека | Ссылки | Отчет о поиске | Индивидуальное задание
