
Pic.1. An elementary cell the of asymmetric membrane.
1. diffusion layer
2. porous sublayer
3. porous backing
4. valve in backing.
Dividing capacity of the membrane is supposed to be characterized by the meaning of selectiveness R:
R = ((C2 - C2')/C2)*100
C2 being a portion of retained substance in j0,%;
C2’ a portion of the same substance in inletting current j’,%.
Moreover, one can characterize dividing capacity of membranes by dividing factor quantity. This quantity is the ratio between permeating through the membrane currents of two components of a divided system.
F = J1/J2
J1 –consumption of air permeating through membrane, m3/min
J2-consumption of nitrogen retanting from the membrane, m3/min.
Alongside with the given characteristics of the membrane dividing is determined by a permeating coefficient ?:
ð= Q*á/S*t*(p1-p2)
where Q is the volume of the permeated through the membrane gas, m3;
á is the membrane thickness, m;
S is the membrane square, m2;
t is the permeation time, c;
p1, p2 are the pressure on different sides of the membrane, MPa.
According to theoretical research, diffusion intrusion through fine membranes is a complicated process consisting of the following stages:
gas absorption on the membrane surface;
gas dissolution on the membrane surface;active gas diffusion through the membrane;
gas emission from the solution on the opposite surface;
gas disorbtion from the reverse side of the membrane
It is considered that gases permeate through membranes in the molecular form, different gases having different permeability degrees.
Gas dividing moving force on polymer membranes, difference of gas mixture component, partial pressure between the both sides of the membrane which can be shown for i-components as:
Pi=Pb*xi*Pí*yi,
where Pb, Pí are gas mixture pressures under and above the membrane, MPa;
xi, yi are parts of i-components in the initial mixture and in the product correspondingly, %.
The moving force characterizes permeability speed of i-component through the membrane, which is defined from the formula
Vi=Pi*Pi
The research of gas mixture division with the help of membranes resulted in creation of different methods of gas dividing process calculation. Using asymmetrical membranes characterized by high gas permeability, the moving process of the divided flow approaches the piston one. In this case the speed of the divided gas flow in interelement space reaches high value which decreases reverse mixturing and longitudinal molecular diffusion directed against the divided flow. The material balance equation of the general composition of membrane apparatus binary mixture is shown as:
Gí=Gk+g,
where Cí is the general consumption of binary mixture, m3/s;
Gk is retant consumption, m3/s.
g is permeate consumption, m3/s.
The analysis of the equation shows that to determine the general consumption of the binary mixture at the given retant consumption one should know permeate consumption. Permeate consumption is determined by the differential equation system calculation which describes the material balance for the membrane apparatus element square suggested in the paper [2]:
-d(gx)=q0*p*dS*(x-ry)
-(g(1-y))=q0*p*dS*(1-x-r(1-y))
where y is a portion of the concluded away gases in permeate, %;
x is a portion of the initial mixture, %;
qc, qf is permeability as to conducted away gas and phlegmatization correspondingly , m3/(m2*s*MPa);
r is ratio of pressure value under and above the membrane;
P is the pressure on the membrane, MPa;
S is the membrane square, m2.
For the case of gas mixture division given with the constant consumption and conducted away gas portion in it and also immutable permeability of the membrane apparatus the system of equations is shown as:
yg=q0*p*dS*(x-ry)
g(1-y)=q0*p*dS*(1-x-r(1-y))
The relation of the permeating phlegmatizer consumption and conducted away mixture contents made from the equation system is shown as:
g=q0*p*S*(1-r)/F(1-y)+y
However oxygen and other conducted away gases concentration in retant but not in permeat can be considered as defining in case of emergency section surroundings inertization. The connection of these concentrations is described by oxygen material balance equation in the process of gas division.
Gïx=Giz+gy
where z is oxygen and other gases volume portion in retant.
Using the equation 10 and 6, one can also define permeating gas mixture consumption:
g=Gi(x-y)/y-z
comparing equation 8 and 11 the relation of oxygen and other gases portion in permeat and this mixture portion in retant can be shown as:
y=(F*Gi(x-z)+xq0PS(1-r))/(Gi(F-1)(x-z)+q0PS(1-r))
However solving practical tasks concerning inert surrounding creation in emergency sections in mines, one should define the number of membrane apparatuses for productive gas getting under given extra pressure on the membrane which is equal to the pressure in the pneumonet.
Consequently, it is necessary to make the relation of membrane apparatuses productivity as to retant to permeation square and pressure on the membrane having the necessary oxygen and other gases portion in retant. Such relation is shown in this equation:
Gi=q0*P*S(1-r)(x-y)/(x-z)(yF-y-F)
Using formulas 6, 11, 12, 13 with the known oxygen concentration one can define the necessary mixture consumption supplied on the device membrane to get necessary retant consumption. Thus, in the result of this equation system calculation which describes the material balance of membrane gas dividing process, one can get the dependence of permeated gas mixture consumption on membrane permeability and pressure on it. The obtained dependence makes it possible to calculate initial gas mixture consumption which is necessary to be supplied on the membrane to get particular productive gas consumption and also define the membrane square depending on the device operation conditions.
Experimental parameter definition and operation conditions adjustment of a shifting mine membrane gas dividing device was held in laboratory and natural surroundings. The device (picture 2) consists of a compressor, dust measures, air moistures, air clearance fibre filter , MGA-20/0,9 dividing modulus, a pipe system with locking slide –valves, manometers, nipples for getting samples and rotameters. The main operation principle is a selective membrane air division into two flows enriched with oxygen and nitrogen correspondingly. To permeate through the membrane the air is given from the compressor under extra pressure.
In this case consumption of the air incoming on the entry of gas dividing apparatus, permeate and retant consumption and also oxygen portion in them are measured.
The technique of experimental research was as follows. The air incoming from the compressor going through the special measure was enriched by coal dust containing 80% particles sized 75 mkm, with the volatile substances outlet 26…28 and ash content 10%. In this case dustiness of the air on the measure outlet was equal to 45…50 mg/m3. Extra moistened to 90-95% air came into the fibre filter, then after refining it was sent into the membrane gas dividing apparatus MGA –20/09 through the pipe-line system and was divided in two sections into the flow enriched with nitrogen. In the process of the experiment one measured consumption and the pressure of the air flow incoming on the membrane apparatus, oxygen and nitrogen consumption and portion in the permeate and retant. To measure the air, permeate and retant consumption one used rotameter RM-40G.the oxygen portion in the flows was determined by oxymeter “Oxycom25D” made by the firm “Dregerverk” and also was controlled by chromatographic method in laboratory conditions.
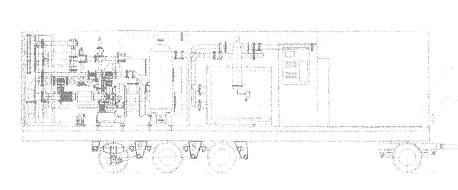
Pic.2. The diagram of the membrane air division mine device
1. compressor;
2. dust measure;
3. air moisturer
4. air clearance fibre filter;
5. locking valve;
6. locking-slide valve;
7. air monometer;
8. membrane air dividing module;
9. nipples of samples getting.
The first series of the experiments were held to adjust the device operating conditions and determine the indication accuracy of the control-and-measurement equipment. In this case the air pressure and productible gas portion changed.
As it is seen from the given data, the deviations in the results of oxygen portion defining in retant got with the help of oxymeter and chromotographic method differ very little. The oxymeter showing too high data, their deviations are not more than +8,3%. Besides, one should mention that when the incoming flow pressure rises the oxygen portion in retant decreases, but the nitrogen one increases. Thus, at the pressure 0,5Mpa on the researched device the oxygen portion in retant equals 6,0% and the nitrogen one is 93,5%.
The adjustment of the device operating conditions showed that the control-and-measurement equipment, gas dividing module and gas pipe-line armature operated in the normal conditions. The optimal pressure which equaled 0,325…0,37 MPa was found. It allowed to keep the compressed air consumption from 0,37 to 0,495 m3/min while providing the necessary operation safety. In these conditions the device operated for about 320 hours. And single measurements of gas dividing device parameters were made. They showed that under the chosen operation conditions the device provided oxygen volume portion in retant 11,5…13,0% or 88,5…87% nitrogen one.
It should be noticed that at the very beginning of our research work (experiments 1-12) the oxygen portion in retant was changing in limits of 11,5…12,5% and then started to grow and at the end of operation it raised up to 13%(experiments 28…36). Later work conditions of gas dividing modulus were changed and MGA-20/0,9 apparatus had been working for a long time providing a considerable amount of nitrogen which was equal to 99,0; 97,0 and 95,0% retant consumption being 0,5;1,0 and 4,15 m3/min correspondingly. Along with 168 hours of continuous work didn’t influence gas dividing parameters.
Adequacy estimation of experimental data based on the above given calculations makes it possible to consider the application of the membrane device as an expedient one to divide mine air for getting nitrogen directly in the mine condition. This estimation can be done with the help of mathematical statistics elements and the theory of reliability.