INTRODUCTION
Wastewater treatment is an important process in modern day society. Anaerobic and aerobic bacteria are used to naturally remove pollutants and impurities contained in the incoming wastewater. Studies have shown that compounds such as toluene, heavy metal ions, ammonia and sodium chloride greatly inhibit the effectiveness of bacteria involved in treating wastewater because they are toxic to the bacteria (Nahar et al., 2000; Sin et al., 2000; Lee et al., 2000; Pollice et al., 2000). Wastewater treatment could be improved if these compounds could be removed before the water is treated by the bacteria.
In the form of flocculants or resins, polymers are actively used in wastewater treatment (Liu et al., 2000; Annesini et al., 2000). Used in the purification of enantiomers, the method of imprinting polymerization offers promise to solving the issue of efficiently treating wastewater. In this case, the impurities present act as the template for the imprinting polymerization. The stability of the imprint will be insured by crosslinking. The wastewater will be purified by means of filtration with the imprinted polymer and, upon removal of the impurities, the polymer will ultimately be reused.
Studies involving the removal of cadmium chloride (CdCl2) using imprinted poly(acrylamide) are being conducted. Officially accepted by the U.S. Food and Drug Administration (FDA) for use in filtration membranes for water purification, poly(acrylamide) has been proven environmentally safe (Denizli et al., 2000). Facilities may also treat wastewater containing hydrophobic contaminants. For the imprinting polymerization with hydrophobic contaminants microemulsion polymerization is used. The imprinting polymerization of poly(methyl methacrylate) in a microemulsion system consisting of water and sodium dodecylsulfate (SDS) is examined.
EXPERIMENTAL
The radical polymerization of polyacrylamide is initiated by the UV activated (256 nm) water soluble radical initiator AAPD (2,2-Azobis(2-aminopropane)-dihydrochloride) and polymerized under nitrogen at room temperature. The cross linker is the hydrophilic ethylene glycol diacrylate. 0.5, 0.3 and 0.1 mol% of cross linker were used. The initial impurity is cadmium chloride. With use of column chromatography, impurity removal efficiency is quantified. Packed with imprinted poly(acrylamide), the impurity is rinsed from the polymer with water and the removal of CdCl2 is ensured by measuring the CdCl2 concentration that is removed. Retention efficiency of the imprinted polymer can then be measured by introducing known concentrations of CdCl2 into the column. The collected liquid is then analyzed by flame atomic absorption.
The microemulsion polymerization is initiated by the water-soluble initiator potassium persulfate (KPS) and heated to 70°C for one hour under constant stirring under nitrogen. The polymer is precipitated out of the microemulsion using ethanol. Once the polymer is filtered, rinsed and dried, IR, NMR, and TGA analysis was conducted. The polymer yields for poly(methyl methacrylate) without cross linker was 80%, with cross linker was 74%.
RESULTS AND DISCUSSION
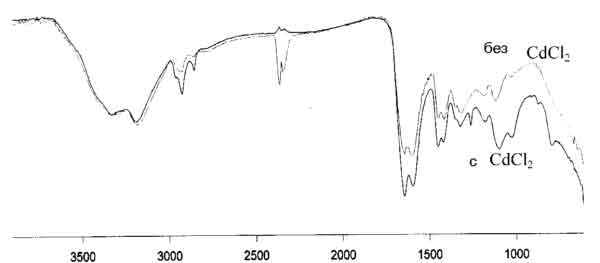
The FT-IR data shown in Figure 1 shows that it is possible to polymerize polyacrylamide in the presence of cadmium chloride. Crosslinking with three different concentrations of cross linker has been performed. 0.5 mol% of ethylene glycol diacrylate resulted in a polymer from which the imprinting molecule could not be removed. Therefore, 0.1 mol% was used in the following study. Molecular weight studies show that the molecular weight increases with cross linking. When the cross linking reaction is performed in the presence of CdCl2, a fraction of the monomer only polymerizes to low molecular weight oligomers. This resulted in a polymer that was dissolving when the imprinting molecule was used. Therefore, current studies are performed with 0.3 mol% cross linker.

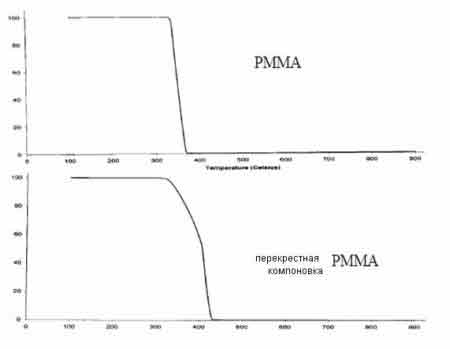
For the microemulsion polymerization with the anionic surfactant sodium dodecylsulfate (SDS), FT-IR and TGA (Thermal gravimetric analysis) data have confirmed the polymerization and crosslinking of poly(methyl methacrylate) (Figure 2).
Further studies are being conducted to assess the efficiency involving the introduction of a contaminant into the microemulsion system. In using Mercury(II) diphenylthiocarbazone as the contaminant, the anionic sulfate headgroups of the surfactant molecules comprising the SDS micelles may interact with Hg2+, inhibiting the imprinting process within the micelles. Thus, nonionic surfactants are being examined currently. The molecular weight and the porosity of the polymer will be optimized for impurity removal.
CONCLUSIONS
Imprinting polymerization for hydrophilic and hydrophobic impurities is being developed for the use in wastewater treatment. Molecular weight, crosslinking density and porosity are being optimized. The random polymerization of a variety of acrylates and acrylamides will be attempted to increase the removal efficiency.
Ññûëêè
1. Nahar, N., Alauddin, M. and Quilty, B. World J. Microbiol. Biotechnol. 2000, 16, 307-311.
2. Sin, S.N., Chua, H., Lo, W. and Yu, P.H.F. Appl. Biochem. Biotechnol. 2000, 84-6, 487-500.
3. Lee, S.M., Jung, J.Y. and Chung, Y.C. Biotechnol. Lett. 2000, 22, 991-994.
4. Pollice, A., Rozzi, A., Tomei, M.C., Di Pinto, A.C. and Limoni M. Environ. Technol. 2000, 21, 535-544.
5. Liu, Y., Wang, S. and Hua, J.J. Appl. Polym. Sci. 2000, 76, 2093-2097.
6. Annesini, M., Gironi, F. and Monticelli, B. Wat. Res. 2000, 34, 2989-2996.
7. Denizli, A., Say, R. and Arica, M.Y., J. Macromol. Sci. A: Pure Appl. Chem. 2000, 37, 343-356.