OXIDIZING AGENTS
Requirements
Oxidizing agents are usually oxygen-rich ionic solids that decompose at moderate-to-high temperatures, liberating oxygen gas. These materials must be readily available in pure form, in the proper particle size, at reasonable cost. They should give a neutral reaction when wet, be stable over a wide temperature range (at least up to 100°C), and yet readily decompose to release oxygen at higher temperatures. For the pyrotechnic chemist's use, acceptable species include a variety of negative ions (anions), usually containing high-energy Cl-O or N-O bonds:
C1O3 - chlorate ion
ClO4 - perchlorate ion
CrO=4 - chromate ion
O= - oxide ion
Cr2O7= - dichromate ion
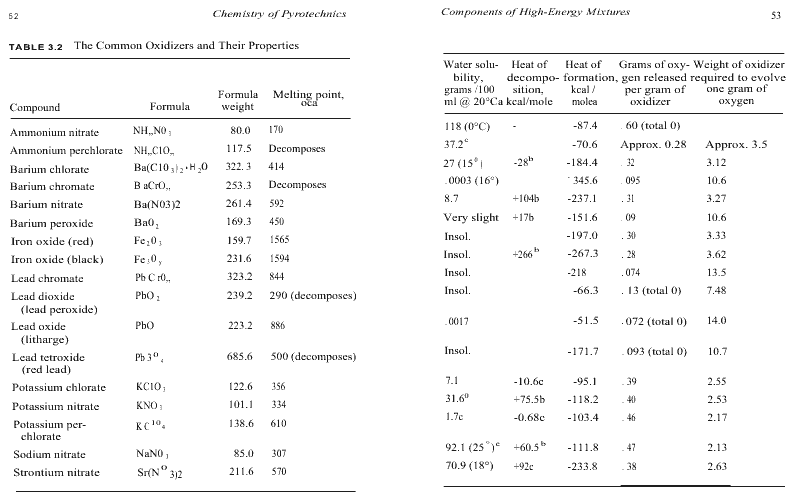
- The oxidizer must be quite low in hygroscopicity, or the tendency to acquire moisture from the atmosphere. Water can cause a variety of problems in pyrotechnic mixtures, and materials that readily pick up water may not be used. Sodium compounds in general are quite hygroscopic (e.g., sodium nitrate - NaNO3 ) and thus they are rarely employed. Potassium salts tend to be much better, and are commonly used in pyrotechnics. Hygroscopicity tends to parallel water solubility, and solubility data can be used to anticipate possible moisture-attracting problems. The water solubility of the common oxidizers can be found in Table 3.2. However, it should be mentioned that large quantities of sodium nitrate are used by the military in combination with magnesium metal for white light production. Here, strict humidity control is required throughout the manufacturing process to avoid moisture uptake, and the finished items must be sealed to prevent water from being picked up during storage.
- The oxidizer's positive ion (cation) must not adversely affect the desired flame color. Sodium, for example, is an intense emitter of yellow light, and its presence can ruin attempts to generate red, green, and blue flames.
- The alkali metals (Li, Na, K) and alkaline earth metals
(Ca, Sr, and Ba) are preferred for the positive ion.
These species are poor electron acceptors (and conversely,
the metals are good electron donors), and they
will not react with active metal fuels such as Mg and Al.
If easily reducible metal ions such as lead (Pb+2 ) and
copper (Cu+2) are present in oxidizers, there is a strong
possibility that a reaction such as
Cu(NO3)2 + Mg -> Cu + Mg(NO3)2
will occur, especially under moist conditions. The pyrotechnic performance will be greatly diminished, and spontaneous ignition might occur. - The compound must have an acceptable heat of decomposition. A value that is too exothermic will produce explosive or highly sensitive mixtures, while a value that is too endothermic will cause ignition difficulties as well as poor propagation of burning.
- The compound should have as high an active oxygen content as possible. Light cations (Na+, K+, NH+ ) are desirable while heavy cations (Pb+2, Ba+2 ) should be avoided if possible. Oxygen-rich anions, of course, are preferred.
- Finally, all materials used in high-energy compositions should be low in toxicity, and yield low-toxicity reaction products.
and the use of Teflon with magnesium metal in heat-producing mixtures,
In both of these examples, the metal has been "oxidized" - has lost electrons and increased in oxidation number -- while the carbon atoms have gained electrons and been "reduced."
Table 3.2 lists some of the common oxidizers together with a variety of their properties.
ДонНТУ|Портал магистров ДонНТУ|||назад в библиотеку