Source of information: http://www2.mbari.org/~coletti/dropbox/Optode/Sea_Tecnology_Feb_2003_Optodes.pdf
Absolute Oxygen Concentrations with High Precision without Repetitive Calibrations; Long-Term Stability
Since most biological and chemical processes in some (i way involve oxygen, it is the single most important parameter to measure in marine biology and chemistry. For environmental reasons, it is also critical to monitor oxygen in areas where the supply is limited compared to the demand (e.g., fjords, around fish farms, in shallow coastal areas, in lakes and rivers, in waste-water treatment plants, etc.).1 Oxygen can also be used as a tracer in oceanographic circulation studies, if the accuracy of the measurements is high enough. Accurate in-situ measurements of oxygen have, until today, been difficult since the existing techniques (electrochemical sensors) are functionally limited. In this article, we briefly describe and discuss different methods to measure dissolved oxygen plus the parameters that are affecting the measuring principles, followed by examples of field data from recently developed oxygen optodes.

Oxygen Measuring Methods
Winkler Titration. The classical method to analyze oxygen content in water is a two-step chemical precipitation of the dissolved oxygen followed by a titration. First described by Win-kler in 1888, the method has remained the absolute standard method until today— it is used in all applications where a high measurement accuracy is needed. The method is also routinely used to calibrate and verify the functioning of dissolved oxygen sensors. Winkler titration, a laboratory method, can only be performed on collected water samples. It involves several steps of time-consuming wet chemistry; therefore, it is not a suitable method to obtain data with high spatial and temporal resolution. In addition, the accuracy and precision of Winkler titration is dependent on the experience and meticulousness of the person doing the work, as well as on the quality of the chemicals used and the titra-tion equipment.
Electrochemical Sensors. For in-situ measurements of oxygen, electrochemical sensors (often called Clark type sensors after a U.S. patent in 1959)2 have been used for more than four decades. The functioning principle is that dissolved oxygen diffuses through a membrane and reaches a cathode where the oxygen is reduced. The electric current between the cathode and an anode is measured and the magnitude of this current is correlated to the concentration of oxygen in the surrounding solution. All electrochemical sensors consume oxygen, although the consumption can be reduced if using pulsed or small size sensors (e.g., micro-electrodes).3
Several manufacturers and users of electrochemical oxygen sensors test and calibrate their equipment by varying only the oxygen concentration. This normally gives a linear relationship between sensor response and oxygen concentration.
It is, however, well known that these sensors are influenced by changes in other parameters as well. Multivariate statistical methods would be needed to determine how other factors influence the response of a sensor. Such methods are common when evaluating complexly interrelated data from biological experiments or industrial processes. The behavior of one type of electrochemical oxygen sensor was tested in such a multivariate way for response in oxygen concentration, relative to changing temperature, salinity, pressure, stirring and pH.4
The only factor that did not have an influence on the sensor response was pH; all the other parameters biased the sensor readings in complexly interrelated ways.
In addition, every sensor was individual and the behavior of a sensor changed when membranes and electrolyte were exchanged. It is also known that the electrolyte and membranes used on electrochemical sensors age over time (weeks to months) in a way that is difficult to predict.
Therefore, the major conclusion from these and other investigations is that the use of electrochemical sensors to measure oxygen in the aquatic environment, especially over longer times, remains a semi-quantitative method.

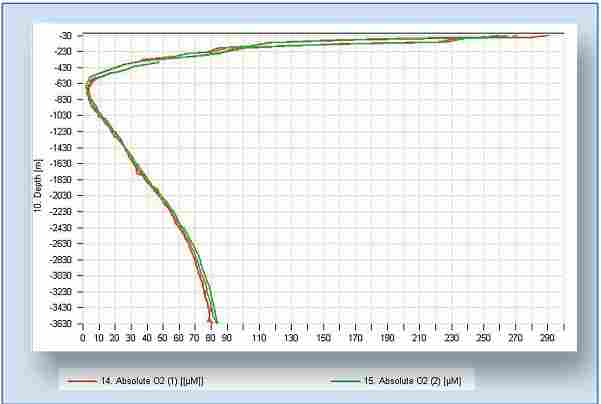
Three weeks of comparative measurements with an optode and with an electrochemical sensor. Two oxygen profiles done to 3,600 meters. Both the up and down casts are shown.
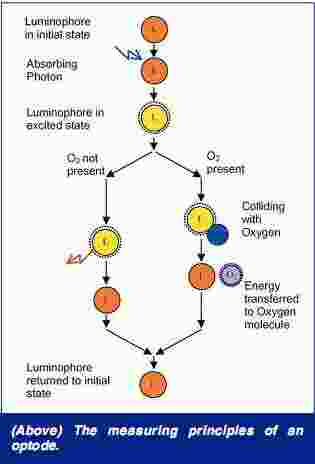
Optodes. Lifetime-based optodes offer a better-adapted principle to monitor absolute oxygen concentrations. The measurements are based on the ability of selected substances to act as dynamic fluorescence quenchers. For example, with oxygen, if a ruthenium-complex is illuminated with a blue LED, it will be excited and emit back a red luminescent light with an intensity, or lifetime, which directly depends on the ambient oxygen concentration.
The principle of fluorescence quenching has been known for decades, but it is only recently that these methods have been used in aquatic applications. It is important to distinguish between two different measuring principles, which are intensity (how strong the returning red light is) and lifetime (how quickly the returning red light disappears). Intensity-based measurements are technically easier to realize, but they can drift over time. Lifetime-based measurements are considered superior for both long-term (no drift) and fast-response applications.5-10
A multivariate calibration exercise, similar to that of the electrochemical sensors, was performed with lifetime-based optodes. Stirring had no effect since the sensor does not consume oxygen. This is a major advantage and explains why optodes are less sensitive to fouling than electrochemical sensors (which do consume oxygen) and need good water circulation in front of the membrane. The effect of pressure was to lower the response about three percent per 1,000 meters of water depth, but this effect is fully reversible and fully predictable. Electrochemical sensors often suffer from irreversible pressure effects (hysteresis) that can often be noticed when profiling from the surface down into the deep sea and back again. Temperature also has an influence on the optical measurements, but since the optodes described here are equipped with temperature sensors, this effect is automatically compensated for by the internal processor.
As with electrochemical sensors, salinity also influenced the response of the optode, but in a straightforward way without the complex behavior shown by the electrochemical sensor. Consequently, in environments where the salinity is varying more than one part per thousand, it has to be measured in parallel, and the oxygen readings have to be compensated for these variations by a simple formula.
In contrast to electrochemical sensors, lifetime-based optodes do not show any signs of aging (within periods of three years) and, consequently, there is no need for repetitive recali-brations. Hydrogen sulfide (H2S) might damage an electrochemical sensor but not an optode, which make optodes also suitable to use in suboxic and anoxic environments.
The lifetime-based optical principle makes it possible to measure absolute values of oxygen without recalibra-tions. Data have shown that optodes have a similar accuracy and precision, provided that compensation is done for salinity and pressure, as with Win-kler titration. More data is, however, needed to verify this statement.
Optode Description
In a joint effort, Aanderaa Instruments A/S and Precision Sensing GmbH (PreSens) have developed a lifetime-based oxygen optode. Pre-Sens has developed the oxygen-sensitive foils and Aanderaa the electronics and the optical system. The sensor has its own digital signal processor that treats the signal, compensates for the calibration constants and gives out absolute readings of oxygen in micro-moles per liter (easily converted to milligrams per liter by a division with 31.25), percent saturation and temperature. As an option, the sensor can also output raw data.
The “oxygen saturation formula,”11 with respect to temperature, is preprogrammed into the sensor processor flash memory. An incorporated thermistor measures temperature that is used to correct the measurement for temperature variations, which affects both the responses of the sensor foil and the oxygen saturation in the water.
Emphasis has been put into making a small, robust sensor that can be used everywhere in the aquatic environment.
The titanium sensor housing is 36 millimeters in diameter and 86 millimeters long. The pressure rating is to 6,000-meter water depth. More information about the sensor is available at www.aanderaa.com.
Field Data
Influence of Fouling. Since optodes do not consume oxygen, are not stirring sensitive and have foils with long-term stability (tests have shown that they are stable for years), lifetime-based optodes are well suited for long-term monitoring on moored instruments.
As an example, measurements were taken off a pier at Scripps Institution of Oceanography by two sensors and compared. Both sensors showed similar responses to natural changes in oxygen until a large chunk of kelp got stuck on the instrument and prevented an accurate water circulation in front of the Clark sensor. The response of the Clark sensor then rapidly decreased. The optode was never affected by the kelp.
There are several other examples, both from fresh water and marine applications, where a similar behavior has been noticed.
In the rivers around Paris (River Orge), France, the authorities (SIVOA responsible, Bouchy) have installed a permanent system of stations that monitor the environmental conditions in the rivers online.
One of the most important parameters to monitor at these stations is oxygen, which has been done with electrochemical sensors that are cleaned and recalibrated weekly.
In these freshwater systems, the drift of the electrochemical sensors is created by a layer of slime that restricts water circulation in front of the membrane and leads to a drop in the sensor signal. An optode was installed in parallel with the electrochemical sensor at one of these sights for a three-week test period.
The conclusion from this test was that the electrochemical sensor, in reality, needed cleaning every second day to give reliable data. After cleaning, it has to be recalibrated with a two-point calibration. The optode was never affected by the biofilm—it showed no signs of signal drift during the whole three-week test period.
There are, however, limitations when fouling will start to have an influence on the optode readings. Such situations occur when the fouling consists of organisms that change the local oxygen conditions in front of the foil. Typically, such situations can occur in the marine environment when, for example, barnacles have grown on the sensor membrane.
When measuring with an unprotected optode in the Chesapeake Bay (United States) at a water depth of one meter, the fouling started to have an effect on the sensor response after approximately seven days. A copper net (used for domestic cleaning) was then wrapped around the sensor, which eliminated fouling effects on the sensor response for the remaining test time of 40 days.
Deep Sea Profiling. As indicated in multivariate calibrations, optodes are slightly affected by pressure. The pressure effect results in approximately a three percent lower reading per 1,000 meters of water depth.
The pressure effect on an optode is fully predictable; it is reversible and is not related to the age of the sensor foil, as it can be for electrochemical sensors.
In one example, two profiles to a water depth of 3,600 meters were mounted on a RCM11 current meter that was lowered on a cable. The down and up casts were similar and the two sensors matched well.
Furthermore, neither of the optodes suffered from any irreversible pressure effects since both sensors return to the same readings at the surface.
The response time is generally faster for optodes than for electrochemical sensors of similar size. The 63 percent response time of the here-described sensors is less than five seconds.
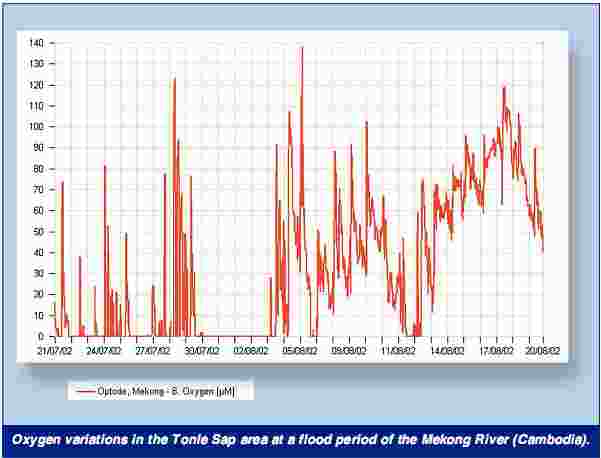
Oxygen Variations. Tonle Sap lake and flood plain is one of the most productive ecosystems in the world, and it is of crucial importance for the Cambodian people as a source for protein and food.
Tonle Sap is a part of the Mekong River basin, and it is flooded annually due to the monsoon rains and the consequent backwater effects in the Mekong Delta.
The flow in the Tonle Sap River is reversed to upstream direction and as a consequence, the Tonle Sap Lake grows five-times bigger compared to the dry season lake. Long-term dissolved oxygen measurements help in understanding the living conditions of the fish.
Large areas of the inundated flood plain become anoxic for extended periods of time during the flood season and affect the movements and migration of fish, occasionally leading to fish mortality. Data from one month of measurements with an optode mounted on a recording current meter (RCM9) were plotted.
The measurement site Phlov Tuk was located in the inundated forest/fields, where the dissolved oxygen declined to very low levels in the beginning of the flood due to the high amount of organic material in the water.
The measurement depth was one meter from the surface in the beginning, and about two meters at the end of the measurement period, due to the rapid increase of the water depth. The RCM9 was fixed to a branch of an inundated tree, hidden away from any unwanted visitors. The dissolved oxygen conditions improve with the rising flood as more oxygen saturated Mekong River water flow onto the flood plain.
Performance Summary
A summary of the performance of the lifetime-based optodes described is:
• high precision and accuracy-optodes measure absolute oxygen concentrations without repetitive calibrations
•long-term (years) stability
• not sensitive to stirring or water movement across the membrane
•low sensitivity to fouling
• predictable and reversible effects of pressure (rated to 6,000 meters)
•fast response time
• the same technique can be used for measuring other substances12
• optodes can be used in 2D applications
References
1. Diaz, Journal of Environmental Quality, 30, 275-281, 2001.
2. Clark, U.S. Patent number 2,913,386, 1959.
3. Revsbech, Limnology and Oceanography 34, 474-478, 1989.
4. Berntsson et al., Analytica and Chimica Acta, 355, 43-53, 1997.
5. Wolfbeis, Fiber Optic Chemical Sensors and Biosensors, Volumes I and II, 1991.
6. Klimant et al., Limnology and Oceanography, 42, 1,638-1,643, 1997.
7. Holst et al., Sensors and Actuators B, 38-39, 122-129, 1997.
8. Demas et al., Analytical Chemistry News & Features, December 1, 793-800, 1999.
9. Glud et al., Deep-Sea Research, 46, 171-183, 1999.
10. Stokes and Romero, Limnology and Oceanography, 44, 189-195, 1999.
11. Garcia and Gordon, Limnology and Oceanography, 37, 1,307-1,312, 1992.
12. Huber et al., Analytica and Chimi-ca Acta, 449, 81-93, 2001.
13. Glud et al., Limnology and Oceanography, 46, 2,073-2,080, 2001.