Èñòî÷íèê:
http://www.hachenvironmental.com/web/ott_hach.nsf/gfx/ C625B62B79697D9FC125766B002A747D/$file/Hach%20Env%20White%20Paper%20-%20LDO.pdf
Àâòîð: Dr. Thomas O. Mitchell, PhD
Luminescence Based Measurement of Dissolved Oxygen in Natural Waters
Abstract
1. Introduction
Oxygen quenching luminophores have been studied from at least 1939 when Kautsky1 described quenching of luminescence by oxygen. More recently, as optical sources, detectors, and data processing have become more advanced, the application of luminophores to the measurement of oxygen concentrations in liquids has resulted in benchtop instruments and optodes2, with significant advances made in the 1990’s3. Recent advances in blue light-emitting diodes and low-powered high-speed electronics have enabled the miniaturization of oxygen sensitive optodes to the point of field-deployable units. The sensors do not consume oxygen and are stable over long deployment periods. The sensors have rapid response times, typically with t0.63 of less than 60 seconds, often less than 30 seconds for changes as high as 8 mg/l. The sensors have predictable temperature dependencies that are corrected using a local temperature sensor.
Hach Company has intensively studied the application of oxygen quenched luminophores for water quality analysis since 1997. Hach scientists recognized that the oxygen quenching luminophores were extremely well suited for water quality analysis, and set out to overcome two of the most significant challenges for developing this technology into a commercially available product – protection from photobleaching so that the sensor could be used for extensive periods of time and in field environments, and repeatability of the printing process such that the luminophore is consistently and affordably integrated into a sensor cap.
Today, Hach LDO® sensors are designed, manufactured, and serviced at Hach Company headquarters in Loveland, Colorado, allowing the company to maintain complete control over the entire application of the technology. During product development of Hach LDO, Hach engineers developed and patented (US Patent #6,912,050) a unique algorithm that allows phase shift measurement with minimal exposure to potentially harmful light. This measurement scheme limits exposure of the luminiphore to the excitation LEDs such that the expected life of the luminiphoreextends well beyond 1 year, even with heavy use. Hach LDO was first made commercially available in 2003 in an application designed for the process industries. In 2004, Hach re-packaged the Hach LDO technology into a compact sensor that is ideally suited for use in a laboratory environment. In 2005, the Hach LDO sensor was completely integrated into Hach Environmental’s Hydrolab Series 5 family of multi-parameter sondes.
The remainder of this paper will discuss the process by which dissolved oxygen is measured in three accepted methods – Winkler Titration, Clark Cell Electrodes, and Luminescence Based Optodes. The strengths and limitations of each method will be covered, and multiple field and laboratory data sets will be presented to quantify the performance of Hach LDO compared to the other available technologies.
2. Accepted Methods of Dissolved Oxygen Measurement in Water
2.1 Winkler Titration
The Winkler titration procedure4 is the first recognized method for determination of oxygen concentrations in natural waters. The technique is a destructive chemical titration where aqueous samples are treated with manganous sulfate, potassium hydroxide, and potassium iodide to form manganous hydroxide, Mn(OH)2. Oxygen in the sample reacts with the Mn(II) species giving Mn(III). The Mn(III) is inherently unstable and will further react with another O2 molecule to form the Mn(IV) species. In order to fix the reaction, acidification is used to convert MnO(OH)2 to manganic sulfate which acts as an oxidizing agent to release free iodine, I2. This iodine is stoichiometrically equivalent to the dissolved oxygen in the sample and is titrated with sodium thiosulfate or phenylarsine oxide to its starch indicator endpoint. The Winkler method is subject to numerous interferences such as the presence of nitrite ion, ferrous and ferric iron, suspended solids, and organic matter5.
The method is prone to overreporting DO concentrations in anoxic and underreporting DO concentrations in hyperoxic environments as the aqueous sample and Winkler reagents are exposed to air during the procedure.
2.2 Clark Cell Electrodes
Membrane covered amperometric detectors are commonly used for the measurement of oxygen in natural waters, with most designs following principles described in a fundamental patent awarded to H. A. Clark. Clark was awarded US Patent 2,913,3866, "Electrochemical device for chemical analysis" in November 1959. "Clark cell" designs have a thin organic membrane covering a two-electrode cell, separating the cell and electrolyte solution from the test solution, and keeping a thin layer of electrolyte in direct contact with the cathode. Oxygen diffuses through the membrane and is reduced on the cathode surface:
Î2 + 2Í2Î + 4å- → 4ÎÍ-
The reduction occurs because the cathode is held at a sufficiently negative voltage to reduce the oxygen, with careful consideration to keep the bias voltage sufficiently large to reduce the oxygen but not so high as to reduce other species. The dissolved oxygen in a given sample is calculated by measuring the cathodic current and sample temperature. A relative measure of dissolved oxygen compared to a fully saturated sample is determined using the cathodic current, temperature, barometric pressure, and salinity.
In a Clark cell electrode design, the greater the oxygen partial pressure, the greater the rate of oxygen diffusion through the membrane. Due to consumption of oxygen at the cathode and diffusion dependence of oxygen through the membrane, sufficient flow of fresh water is necessary to maintain accuracy and precision of DO analysis. Other interferences include organic growth or decay that can add or remove oxygen from the water prior to transfer of oxygen through the membrane. In addition, contamination from oils and other polymers can lead to a decrease in diffusion rates, changing the calibration function of the electrode. Some materials used in commercial Clark cell electrodes are susceptible to poisoning by contaminants, which leads to a decreased response. Over time, membranes in Clark cells deteriorate to the point of needing replacement, electrolytic solutions become less pure, and the electrodes are consumed to the point of limited response to oxygen exposure7. All such issues result in the need for frequent servicing and refurbishing of the Clark cell style sensors, with associated material and labor costs.
2.3 Luminescence-based Optodes
FIGURE 1. OPTODE DESIGN
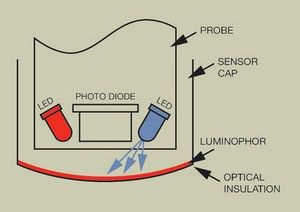
As shown in Figure 1, the luminescent dissolved oxygen sensor’s active optical components consist of a pair of blue and red light-emitting diodes (LEDs) and a silicon photodetector. The sensor cap has a coating of a platinum based luminophor that is excited by the light from the blue LED. The luminophor is coated on the outside with a carbon black polystyrene layer for optical insulation, providing excellent protection against photobleaching from external light sources when the sensor cap is attached to the sensor.
The blue excitation LED is sinusoidally modulated at a frequency related to the luminophor’s luminescence lifetime and the upper and lower lifetimes of analytical interest. The measured parameter of interest from the optode is the phase delay (essentially a time delay) between the exciting blue LED signal and the detected red emission from the luminophor, with the phase delay inversely related to the amount of dissolved oxygen near the luminophor, typically oxygen in the water of interest. This phase-modulation technique is used to measure the lifetime of the oxygen-dependent quenching of luminescence.
The use of the phase-modulation technique means that intensity fluctuations of the blue LED or bleaching effects of the luminophor have no discernable impact on the lifetime measurement throughout the life of the sensor. In addition, because of the inverse relationship between oxygen concentration and phase delay of the emitted red light, the signal-to-noise ratio is particularly advantageous for measuring very low dissolved oxygen concentrations. Finally, the blue and red LEDs are alternatively switched between measurement cycles, allowing the red LED to provide an internal reference for the optical and electronic signal paths8. This internal reference provides measurement stability by correcting for temperature or time induced changes in the phase measurement electronics.
2.3.1 Calibration and Temperature Dependence
The optical quenching of the luminophore is strongly temperature dependent. It is important to measure the temperature with high precision (repetitiveness to a measured temperature) and to closely monitor the temperature of the luminophore sensor cap during the measurement cycle. When calibrating the instrument, it is critical that the luminophore sensor cap be in thermal equilibrium with the water of interest and with the temperature probe measuring the temperature of the water of interest.
For example, when using water-saturated-air for calibration, it is necessary that the luminophore sensor cap and the temperature probe both be completely out of the water and in emperature equilibrium with the water-saturated-air for the calibration of 100% saturation for the probe. Similarly, when using airsaturated-water for calibration, it is necessary that the luminophore sensor cap and the temperature probe both be fully immersed in the water and in temperature equilibrium with the air-saturated-water for the calibration of 100% saturation for the probe. When calibrating the probe in the field, it is recommended to shield the calibration cup from thermal heating effects by using a sun-shield or other method of ensuring temperature stability in the alibration cup throughout the duration of the probe calibration.
3. Results
3.1 Comparison of Hach LDO and Winkler Titration
FIGURE 2. COMPARISON TO THEORETICAL VALUES
Test Description: Using NIST traceable O2/N2 gas mixtures to create equilibrium water samples at known temperature and pressure values, dissolved oxygen measurements from Winkler, Membrane, and Hach LDO (Luminescent) measurements were compared to theoretical DO values (Hitchman, 1978). The Hach LDO measurements show superior accuracy over the entire span compared to the other techniques
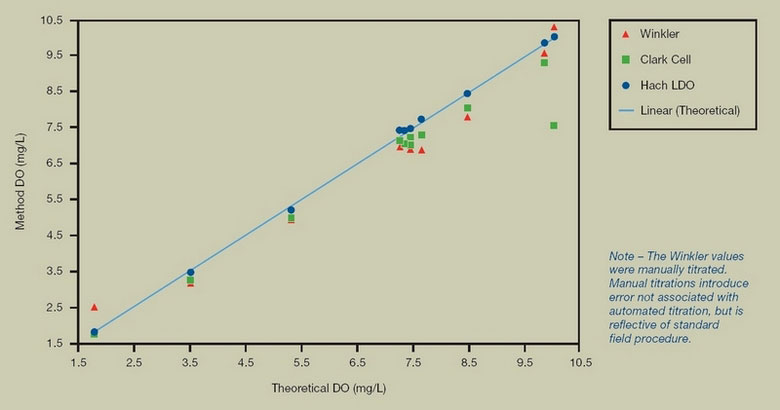
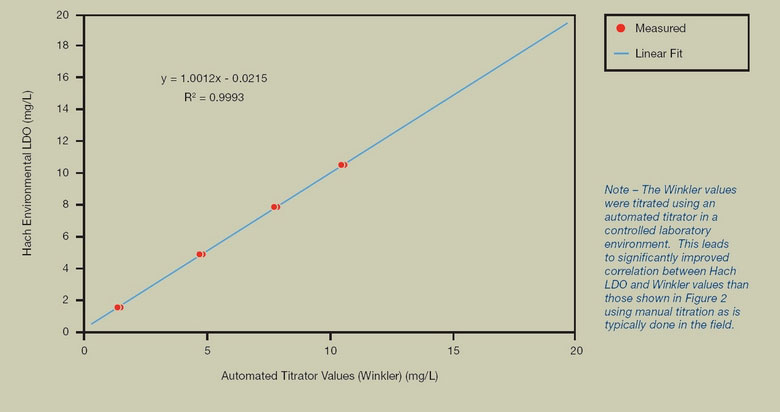
FIGURE 3. COMPARISON TO WINKLER TITRATION DISSOLVED OXYGEN VALUES
Using an automated Winkler titrator, measurements from a Hydrolab Series 5 sonde with Hach LDO were shown to have a high degree of correlation to Winkler values. Each data set consists of two samples, which overlap due to close agreement of the values.
3.2 Comparison of Hach LDO and Clark Cell
FIGURE 4. MEASUREMENTS IN HIGH SALINITY
Test Description: In a controlled laboratory environment, water salinity was modified to the desired level using a commercial sea salt mixture. The tank was depleted of oxygen using a nitrogen purge (decreasing DO concentration) followed by oxygen saturation using an oxygen purge (increasing DO concentration). Hach LDO comparison to membrane dissolved oxygen sensor at mid (6.9 ppt) and high (45.5 ppt) salinities shows agreement well within the error margins of +/- 0.2 mg/l for the membrane sensor and +/- 0.1 mg/l for the Hach LDO sensor (below 8 mg/l) and +/- 0.2 mg/l for the Hach LDO sensor (above 8 mg/l).
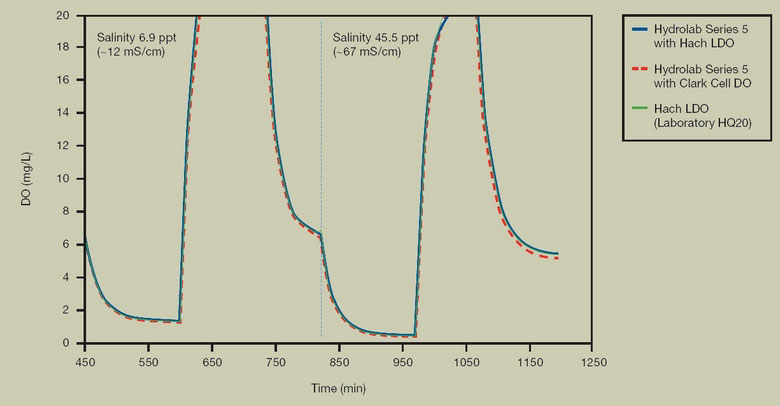
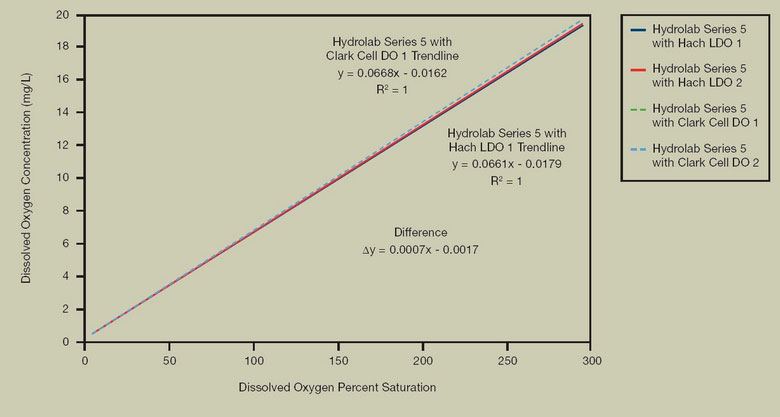
FIGURE 5. PERCENT SATURATION DETERMINATION
Test Description: In a controlled laboratory environment, the water DO level was modified using nitrogen and oxygen. The tank was depleted of oxygen using a nitrogen purge followed by oxygen saturation using an oxygen purge. Several hundred data points were taken, distributed across oxygen concentrations. Hach LDO percent saturation values compared to membrane sensors shows close agreement, demonstrating that percent saturation values calculated from Hach LDO mg/l measurements are equivalent to percent saturation values reported by membrane sensors.
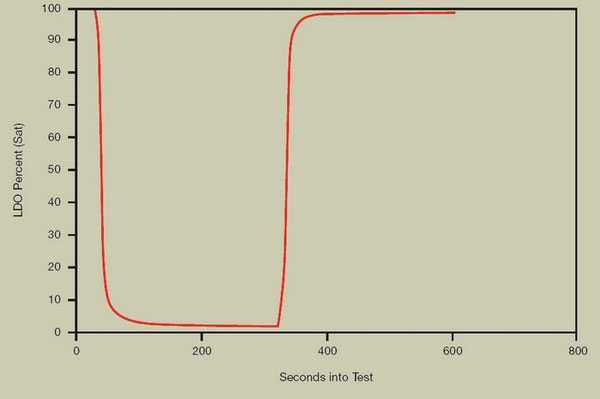
3.3 Response Time Performance of Hach LDO
FIGURE 6. RESPONSE TIME
Hach LDO response time to step change in oxygen concentration is less than 30 seconds to reach t0.95 both when decreasing from 8 mg/l to 0 mg/l and increasing from 0 mg/l to 8 mg/l.
3.4 Field Data Collected Using Hydrolab Series 5 with Hach LDO vs. Clark Cell
FIGURE 7. LOW CONCENTRATION AND TEMPERATURE
Hach LDO comparison to Winkler titration at low oxygen concentration and low temperature shows close agreement, demonstrating the ability to reach true zero and to operate at low temperatures.
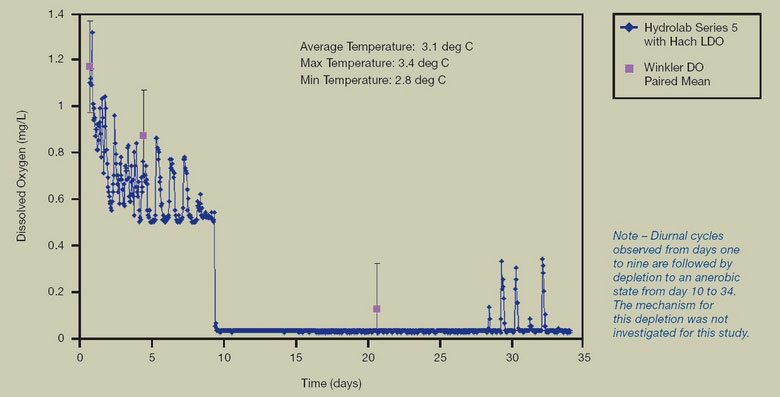
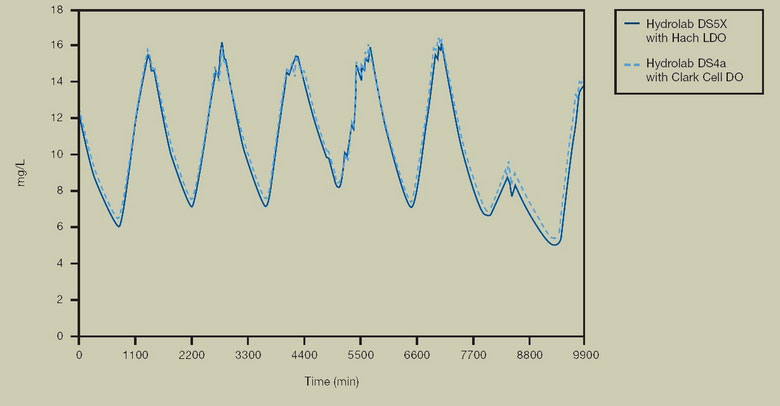
FIGURE 8. ENVIRONMENTAL TESTING
Hach LDO comparison to Clark cell dissolved oxygen measurement in environmental waters. Tests were conducted over a week in a pond near Niwot, CO. Hach LDO and the membrane sensor were within measurement error margins for the entire week, with 15 minute measurement intervals clearly showing diurnal cycles in the eutrophic pond.
4. Conclusions
An overview of three methods for measuring dissolved oxygen in natural waters has been presented.
The Winkler method has strengths with regard to its ability to accurately determine oxygen concentrations in natural waters, but it is limited by its destructive nature and laborious wet chemistry procedure. In addition, it is difficult to measure samples far from equilibrium (i.e. in either anoxic or highly hyperoxic environments) using the Winkler method.
The Clark cell, a membrane covered amperometric sensor, represented a significant step forward in continuous monitoring of dissolved oxygen at its introduction nearly 50 years ago. The Clark cell’s inherent limits due to its consumptive nature and the need for frequent cell maintenance were viewed at that time as necessary inconveniences due to the value of the supplied data.
A luminescent dissolved oxygen sensor using a phasemodulation technique to measure oxygen-dependent luminescence quenching was reviewed. This sensor, a type of optode, offers ignificant enhancements in terms of accuracy and sensor life over other existing technologies used to measure dissolved oxygen, including optodes using intensity-based measurements. It is free from known nterferences at normal interferent concentrations, a significant improvement over Winkler and Clark cell methodologies. With its broad dynamic range, high accuracy, and ability to selfcorrect for temperature and other changes in the sensor electronics, the reviewed LDO sensor proves to be a robust and repeatable standard method for measuring dissolved oxygen in natural waters.
Acknowledgements
The author gratefully acknowledges assistance provided by Russell Young, Cary Jackson, John Lee, Kevin West, Mike Sadar, and Brian Staff, all of Hach Company.
References
H. Kautsky, "Quenching of luminescence by oxygen", Transactions Fariday Society 35, 216-219 (1939).
D. W. Libbers and N. Opitz, "The pCO2/pO2 optrode: A new probe for measuring pCO2 and pO2 of gases and liquids", Z. Naturforsch. 30 (C), 532-533 (1975); I. Bergman, "Rapid response atmospheric oxygen monitor based on fluorescence quenching", Nature 218, 396 (1986); J. R. Bacon and J. N. Demas, "Determination of oxygen concentrations by luminescence quenching of a polymer-immobilized transition-metal complex", Analytical Chemistry 59 (23), 2780 (1987).
D. W. Libbers, "Fluorescence based chemical sensors", Advances in biosensors 2, 215-260 (1992); Wolfgang R. Gruber, I. Klimant, and O. S. Wolfbeis, "Instrumentation for optical measurement of dissolved oxygen based on solid state technology", Ocean Optics 12, 448-457 (1993); B. H. Weigl, A. Holobar, W. Trettnak et al., "An optical triple sensor for measuring pH, oxygen and carbon dioxide in bioreactors", Proceedings of the SPIE - The International Society for Optical Engineering 1796, 287 (1993); I. Klimant, V. Meyer, and M. Kuhl, "Fiber-optic oxygen microsensors, a new tool in aquatic biology", Limnology and Oceanography 40, 1159-1165 (1995).
L.W. Winkler, "The Determination of Dissolved Oxygen in Water", Berlin. Deutsch. Chem. Gellsch. 21, 2843 (1888); L.W. Winkler, "Determination of Oxygen (Addition)", Z. Angew. Chem. 24, 831 (1911); L.W. Winkler, "Sauerstoff-Flasche", Z. Angew. Chem. 25, 1563 (1912).
Standard Methods, 20th ed.
H. A. Clark, Patent No. 2,913,386 (1959).
Michael L. Hitchman, Measurement of dissolved oxygen. (Wiley, New York, 1978), p.255.
G. Holst, R. N. Glud, M. Kuhl et al., "A microoptode array for fine-scale measurement of oxygen distribution", Sensors and Actuators B (Chemical) B38 (1-3), 122 (1997).