Abstract
Contents
- Introduction
- Statement of the problem
- 1. Analisys of the issue in the world practice
- 2. Physical and chemical bases of the process
- 2.1 Theory of electrolysis
- 2.2 Faraday's Law, the underlying electrolysis
- 2.2.1 The first law of Faraday
- 2.2.2 The second law of Faraday
- 2.3 Factors influencing the process of electrolysis
- 2.4 Pulse Electrolysis
- 3. Experimental unit
- 3.1 Review of existing facilities
- 3.2 Methodology of the experiment
- 3.3 Results of the experiment
- 3.4 Analysis of results
- References
Introduction
Electrochemistry as a science emerged at the turn of the XVIII and XIX centuries. It was then that there were certain problems that brought a new level of the theory of electrochemistry. The impetus for the creation and development of electrochemistry, as a science was the creation in 1799 by Italian physicist A. Volta "voltaic pile" - the first in the history of chemical current sources and the experiences of Italian physiologist Luigi Galvani.
Electrochemistry is a relatively young science. Only at the beginning of last century it was found that by passing an electrical current through the aqueous solutions of salts, chemical transformations occur, which leads to the formation of new substances.It is only the beginning of last century there electrochemistry - scientific direction for the study of electrochemical processes in solutions and melts of substances. Industrial applications of electrolysis became possible after the occurrence in the seventies of the XIX century of powerful DC generators.
It should be noted that electrolysis is one of the most important areas in electrochemistry, at one time served as the basis for important scientific discoveries in the field of electrochemistry. [1]
Statement of the problem
Investigate the electrolysis process, to identify the factors that most influence the process. Develop a pilot plant for the electrolysis products and to analyze and optimize its operation. Develop an electronic power supply that will generate pulses of a given frequency in a wide range.
1. Analisys of the issue in the world practice
A variety of methods of hydrogen production is one of the main advantages of hydrogen energy, as it improves energy security and reduce dependence on individual raw materials.
They are:
- steam conversion of methane and natural gas;
- gasification of coal;
- electrolysis of water;
- pyrolysis;
- partial oxidation;
- biotechnology.
Currently, the most accessible and cheapest process is the steam conversion. According to forecasts, it will be used in the initial stage of transition to a hydrogen economy[2].
2. Physical and chemical bases of the process
Transformations that occur when exposed to the substance by electric current is called electrolytic chemical ones. The process of electrolysis is not the same in all cases and depends on several factors - the nature of the electrolyte, such as the electrolytic bath, optimization of the electrolysis process itself.
2.1 Theory of electrolysis
Electrolysis is due to a direct current power, supplied and the energy released in chemical reactions at the electrode. Thus, electrolysis can occur only in environments that conduct electric current.
The energy consumed by electrolysis to increase the gibbs's energy of the system in the formation of desired products, and partly dissipated as heat in overcoming the resistance in the cell and other parts of the chain. Investigating the products exuded from the electrode in the electrolysis of acids, bases and salts, have found that the cathode is always exuded metal and hydrogen, at the anode are the anions or hydroxyl groups, which are changed then.
Let us consider the processes occurring during the electrolysis more specifically. It is known that there are first kind conductors, in which electricity is carried by electrons, and second kind ones, in which electricity is carried by ions. The electrons interact with ions in the ground circuit, where the first kind conductor is bordered by the second kind one. Thus, the electrochemical processes are occured.
Electrochemical processes occurring at the electrodes during electrolysis, above all, will depend on the ratio of electrode potentials of the electrochemical systems. Of the several possible processes there will take place one, the implementation of which is connected with a minimum expenditure of energy. [1]
2.2 Faraday's Law, the underlying electrolysis
2.2.1 The first law of Faraday
In 1832, Faraday found that the mass M of the substance liberated at an electrode is directly proportional to the electric charge q, passing through the electrolyte:
where k - electrochemical equivalent of the substance;
q - electric charge;
I - amperage;
T - time during which a current is passed.
If passed through the electrolyte during the time t, a constant current with a current I. The coefficient of proportionality k is called the electrochemical equivalent of the substance. It numerically equal to the mass of a substance that is released during the passage through the electrolyte of a single electric charge, and depends on the chemical nature of matter.

where z – the valence of the atom (ion) of the substance;
e – electron charge, e=1,062*10-19;
F=eNA– Faraday constant, F = 96485.
2.2.2 The second law of Faraday
Electrochemical equivalents of different substances are, well as their chemical equivalents. Chemical equivalent of an ion is the ratio of the molar mass of the ion A to its valence z. Therefore, the electrochemical equivalent of:
The second Faraday's law is written as follows:
where М - the molar mass of the substance formed (but not necessarily stand out - and it could enter into a reaction
immediately after the formation) as a result of electrolysis;
I - amperage;
Δt- time during which the electrolysis was carried out with;
n - number of electrons involved in the process, for sufficiently large values of the current strength is equal to the
absolute value of the ion charge, who took part in the electrolysis (oxidation or reduction)[2].
2.3 Factors influencing the process of electrolysis
The efficiency of electrolysis is evaluated a number of factors which include: current, voltage, current density, the efficiency of the current source, current output, the output of the substance, the efficiency of electric power (output energy), energy consumption per unit of the product.
Current load on the electrolyzer characterize its productivity. The higher the current flowing through the electrolytic cell, the more product can be obtained by using this cell. There is a tendency to create powerful electrolyzers, designed in some cases hundreds of thousands of amperes (production of chlorine, aluminum, etc.), the voltage on the electrolyzer is made up of several components:
where: U – total voltage on the block;
ea and ek – equilibrium potentials of the anodic and cathodic reactions;
e эл and e диафр – the voltage drop in the electrolyte and diaphragm;
e конт – the voltage drop at the contacts.
The amount of ea-ek is the voltage decomposition. This value corresponds to the consumption of electricity for electrolysis, which goes directly to the change in internal energy of matter.
In the electrolysis tend to reduce the stress on the cell due to the polarization and state of balance ohmic voltage, i.e. components due to the irreversibility of the process. Decomposition voltage due to the nature of the reactant, and therefore can not be changed. The value of Δea and Δek can be changed depending on the nature of the electrochemical reaction occurring at the electrode, by stirring, raise the temperature of the electrolyte, changing the state of the electrode surface and due to other factors.
voltage drop in the electrolyte is expressed by the equation:
where ρ – electrolyte resistivity;
l – distance among the electrodes (without diaphragm);
S – sectional area of the electrolyte.
If electrolysis is accompanied by the formation of gases, the above mentioned expression is not always exactly correspond to the voltage drop in the electrolyte. This is because the electrodes are allocated gas bubbles, which reduce the active cross section S of the electrolyte and lengthen the current path from one electrode to another. This phenomenon is called gas filling, which can be defined as the ratio of the volume occupied at the time of air bubble to the total volume of the electrolytic cell. The effect of gas filling on the electrical conductivity of the electrolyte can be taken into account by using the following expression:
where ρ and ρ0 – respectively, the resistivities of solid and gas-filled electrolyte;
φ – gas filling.
The value of φ can be reduced by increasing temperature, as well as the special arrangement of electrodes, guaranteeing the free removal of gases from the cell.
The voltage drop in the diaphragm was assessed when deciding the role of the diaphragm in the electrolysis. As for the voltage drop at the contacts, this quantity depends on the excellence of contacts, clean contact surfaces.
Efficiency is the ratio of the voltage stress to the decomposition of the total voltage across the bath:
Current density is the ratio of current passing through the electrolyte to the magnitude of the electrode surface, measured in А/сm2. In industry, working with different current densities - from a few hundred А/сm2(electroplating, gidroelektrometallurgii, chlorine) to several thousand А/сm2 (electrolysis of melts, electrosynthesis, etc.)
The magnitude of the current density characterizes the amount of product produced per unit of electrode surface, i.e. performance of the cell. Therefore, if the increase in current density does not cause the fall release of the product of electrolysis, the process of seeking to hold the highest possible current density.
However, when choosing the optimal values of current density in some cases it is necessary to take into account an increase in the cost of the product by increasing the flow of electricity in the electrolysis due to increasing stress with increasing current density. In the electrolysis of aqueous solutions of the reactions of electrochemical oxidation or reduction reaction is accompanied by decomposition of water into О2 and Н2, вrespectively, evolved at the anode and cathode. Since current is passed through the electrolyte is distributed among several processes taking place at this electrode simultaneously:
where: I – the current flowing through the electrolytic cell;
i1 and i2 – current consumption per unit of time on the first and second electrolytic reaction.
In order to take into account the efficiency of electrolysis passed through the amount of electricity to the formation of a product we introduce the concept of current efficiency.
Theoretically, the required amount of electricity - is the electricity amount would be necessary to obtain the number of units of matter, if the process is carried out with 100% output current and voltage equal to the voltage decay. Thus, the output energy can be determined by the formula:
Electricity consumption is usually referred to as a unit of product produced, measured in (W*hour)/kg или (w*year)/kg. To calculate the energy consumption per 1 ton of DC produced product, you can use the following formula:
where: W – DC power consumption, (W*hour)/kg;
U – the voltage on the electrolyzer;
k – an electrochemical equivalent;
η струму – the current output, of a unit;
1000 – transfer coefficient for W*hour in kW*hour.
Power Consumption AC per unit of product can be determined by dividing the flow of direct current electricity for the same amount of the coefficient of conversion of AC to DC.
2.4 Pulse Electrolysis
Often in studies of pulsed electrolysis does not specify what kind of polarizing current densities and electrode potential in question. In general, the polarizing current and potential are non-sinusoidal functions of time. Then, according to the theoretical fundamentals of electrical engineering, we should distinguish the instantaneous values of current density j(t) and the potential E(t) (the value at any given time period), the maximum jmах, Еmах (the largest at any given time), the average for the period T

and current values, the latter defined as

Without specifying what values of a magnitude operates the author, the study does not allow you to analyze the influence of parameters on the deposition mode. For stationary regimes of electrolysis (DC), all values j(t)=Jср=J=J_.
At the same time, the use of certain values of the potential and current density is incorrect. First of all, it refers to the average values of the potential and the polarization that attract to opinion of the kinetics of electrode reactions with an arbitrary form of the polarizing current. The average value of the polarization is only a quantitative measure of the constant component of the electrode active power equal to ηср*Jср; another practical sense ηср value does not exist. The possibility of occurrence of an electrochemical process does not characterize the magnitude of ηср. This is due to the fact that during the period of the pulse current of the electrode potential takes many different values, sometimes significantly different from the average value of Eср. Therefore, only the instantaneous value of Е(t) (in conjunction with the current j(t)) define the energy state of the electrode and thus determine the probability of discharge of certain ions in a given time [5,6]. In connection with this IM software control values of the current [7,8] and the building as well as hold time for this value of E, which corresponds to the discharge of ions required is an important aspect of the development of pulsed electrolysis. In experimental studies, the most necessary dependencies - potential curves recorded by oscilloscope - time and pulse current density - time. Conventional methods of measuring the potential compensation are not suitable for the purposes of pulsed electrolysis, because they give only the mean value.
3. Experimental unit
3.1 Review of existing facilities
There are several basic designs of electrolyzers, which give a fairly high yield of gas at relatively low cost of energy:
- Hydrogen cell C. Meyer (Meyer Сell);
- Hydrogen cell Joe (Joe Cell).
The remaining fuel cells, which are currently in use are made on the basis of the first two, with some changes in the design of the fuel cell, or in his power supply.
Block C. Meyer (Figure 3.1) has a lot to do with electrolysis unit, except that it works with high potential and low current is better than other methods.
The electrodes are made of stainless steel parallel plates that form either flat or concentric structure. Output of gas depends on inversely to the distance between them; patents suggest a spacing of 1.5 mm gives a good result.
Significant differences are in the nutrition of the cell. Meyer uses an external inductance which forms a resonant circuit with the capacitance of the cell - pure water apparently has a dielectric constant of about 5 - to create a parallel resonant circuit.
It rises a powerful pulse generator which, together with the cell capacitance and diode rectifier circuit of the pump. High frequency pulses produces the potential steps up on the electrodes of the cell until it reaches the point where the water molecule breaks down and there is a short current pulse.
A witness team of independent UK scientific observers testified that U.S. inventor Stanley Meyer successfully decomposed ordinary tap water into constituent elements through a combination of high-voltage pulses with an average current consumption, measured only milliamps.
Reported gas was sufficient to show the hydrogen-oxygen flame which instantly melted steel.
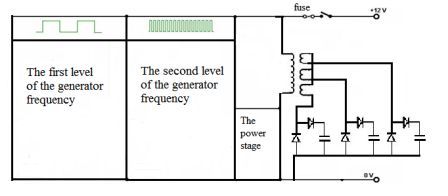
Fig. 3.1 - Stanley Meyer Electrolytic block
Joe cell as well as the Meyer cell is a unique device for obtaining large amounts of hydrogen at the lowest cost of electricity supply can be made from a car battery cell voltages at low DC voltage. The uniqueness of this center is that unlike a cell Meyer, this electrolyser has several tubular resonators of different diameters are inserted into each other (Figure 3.2), and constructive is very simple, but the effectiveness of this device is very high, especially when working cell seen a lot of impressive new effects, such effects are not found in ordinary electrolytic decomposition of water. During the operation of the device that has happened resonators that produce a resonant separation of water, for some reason, very considerably reduce the temperature of the body and the machine is covered with frost, though the ambient temperature does not provide this state, that is, there is a change in the environment field devices. This cooling effect is not yet known, but it is important for understanding not only the resonance technology to produce hydrogen and energy at all[3].

Fig. 3.2 - Joe Electrolytic Block
Search terms modeling the process of decomposition of water into hydrogen and oxygen, which takes place during photosynthesis, led to the simple design of the cell (Figure 2.3), which simulated the annual rings of tree trunks in a gap between the conical electrodes. It turned out that the process of electrolysis can occur at a voltage of 1.5-2.0 V between the anode and the cathode and the current 0.02 A. Therefore, a process called low current.
First of all, we note that the material of the anode and cathode is the only steel, which eliminates the possibility of forming a galvanic cell. However, the cell electrodes a potential difference of about 0.1 in the absence of an electrolytic solution in it. After pouring the solution of the potential difference increases. The positive sign of the charge always appears at the top electrode and the negative - on the bottom. If a constant current source generates pulses, the output of gases increases[4]. Low current electrolysis process may consist of two cycles, one cycle electrolyzer plugged in, and in another - is off[5].
1
Fig. 3.3 – Low amp cell (Patent number 2,227,817)
The experimental setting consists of the cell, regulated power supplies, ammeters and voltmeters.
3.2 Methodology of the experiment
Plate electrodes to dissolve completely, turn the power on and measure the output of gases evolved. The output of gases measured at a current of 2, 3, 4 and 5 amps, and measure the voltage at these values of current power strength.
Experience again for the position of the electrodes A, B and C (Fig. 3.4). The obtained data recorded in the table.

Рис 3.4 – Position of the electrodes
(animation: 9 shots, 5 cycles of repeating, 125 kilobytes)
3.3 Results of the experiment
The experimental results obtained for the three cases (Table 3.1): plate electrodes are divorced, plates and plates of half-built fully erected.
Table 3.1 - Results of the experiment
| № of experience | Current, А | Voltage, V | The volume of gas, ml/min |
| Position of the electrodes А | |||
| 1 | 2 | 2,73 | 10,0 |
| 2 | 3 | 2,93 | 22,2 |
| 3 | 4 | 3,11 | 40,0 |
| 4 | 5 | 3,28 | 60,0 |
| Position of the electrodes B | |||
| 1 | 2 | 2,62 | 12,0 |
| 2 | 3 | 2,73 | 25,0 |
| 3 | 4 | 2,84 | 40,0 |
| 4 | 5 | 2,93 | 66,7 |
| Position of the electrodes C | |||
| 1 | 2 | 2,59 | 6,0 |
| 2 | 3 | 2,69 | 25,0 |
| 3 | 4 | 2,78 | 33,3 |
| 4 | 5 | 2,84 | 50,0 |
Calculate the capacity of current and power density, which is consumed in the formation of 1 ml of gas, according to the formulas:
where: Vг – volume of gas evolved, ml
The calculation results are listed in the table (Table 3.2)
Table 3.2 - Results of calculations
| № of experience | Current, А | Voltage, V | The volume of gas, ml/min | Power, W | Power density, W/ml |
| Position of the electrodes А | |||||
| 1 | 2 | 2,73 | 10,0 | 5,46 | 0,546 |
| 2 | 3 | 2,93 | 22,2 | 8,79 | 0,396 |
| 3 | 4 | 3,11 | 40,0 | 12,44 | 0,311 |
| 4 | 5 | 3,28 | 60,0 | 16,4 | 0,273 |
| Position of the electrodes B | |||||
| 1 | 2 | 2,62 | 12,0 | 5,24 | 0,436 |
| 2 | 3 | 2,73 | 25,0 | 7,19 | 0,288 |
| 3 | 4 | 2,84 | 40,0 | 11,36 | 0,284 |
| 4 | 5 | 2,93 | 66,7 | 14,65 | 0,22 |
| Position of the electrodes C | |||||
| 1 | 2 | 2,59 | 6,0 | 5,18 | 0,863 |
| 2 | 3 | 2,69 | 25,0 | 8,07 | 0,323 |
| 3 | 4 | 2,78 | 33,3 | 11,12 | 0,334 |
| 4 | 5 | 2,84 | 50 | 14,2 | 0,284 |
3.4 Analysis of results
As a result of the experiment were obtained from the dependence the yield of gas current (Figure 3.5) at different positions of the electrodes relative to each other.

Fig. 3.5 - Dependence of the yield gases from the current
According to the schedule dependence of the gas from the current strength can be concluded that the greater the current, the greater the yield of gas in each case. The yield of gas increases in the construction of the electrode to a certain position relative to each other, and then begins to fall. In summary the electrodes with increasing current strength of 2 to 3 amps output of gases increases sharply, but with a further increase of the current growth rate of output of gases is reduced. This can be explained by the fact that on the electrode surface appears a layer of bubbles that do not have time to be removed, thereby reducing the productive area of the electrodes.
To determine the optimum conditions we consider the dependence of the power from the power supply. A plot of power density on the current electrode at different positions relative to each other is shown in Figure 3.6.

Fig. 3.6 - Dependence of power density on the current
With the increase of the current power density decreases, ie decreases the amount of energy that must be expended to obtain 1 ml of gas. Consumption of large amounts of energy at the position of the electrodes and the current in the 2A may be due to the existence of some of the energy barrier to overcome that you need to spend extra energy.
By passing through the electrodes direct current have to spend a lot of energy in the electrolysis. Professor Kanarev0 in his article "The low current electrolysis of water" on December 28, 2003, suggests that if the DC power source will generate a pulse, the output of gases to increase. [4] It was therefore tasked to create a generator that will generate pulses of a given frequency in a wide range.
The basis of the generator was taken by a programmable integrated circuit oscillator LTC6904, which generates a square pulse. Managing IC by using a hardware computing platform Arduino. Arduino board consists of a microcontroller Atmel AVR (ATmega328 and ATmega168 in new versions of the old and the ATmega8) and the binding element for programming and integration with other schemes. The Arduino IDE is a cross-platform applications in Java, which includes a code editor, compiler, and the transfer of the firmware in the module cost. The development environment is based on the programming language C / C++, augmented by some libraries and is designed for programming novices not familiar closely with software development.
Schematic diagram of the apparatus is shown in Figure 4.1. The installation consists of the generator frequency and the power stage of the cell. Schematic diagram of the oscillator frequency is shown in Figure 4.2.
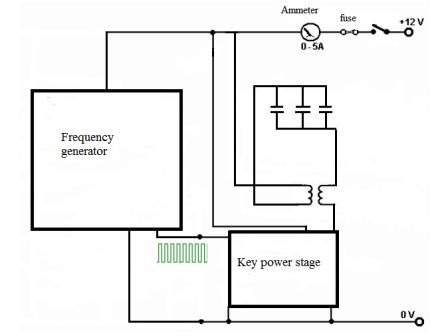
Fig. 4.1 - Schematic diagram of the apparatus
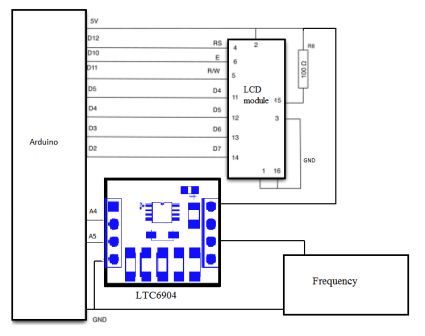
Fig 4.2 - Schematic diagram of the oscillator frequency
At this stage, carried out experiments using a pulsed power supply, and is processing and analyzing the results.
References
- Основы процесса электролиза.[электронный ресурс]. – Режим доступа: http://studyport.ru
- Производство водовода. Материал из Википедии — свободной энциклопедии. [электронный ресурс]. – Режим доступа: http://ru.wikipedia.org/wiki/Производство водорода
- Водородная энергетика - CyberEnergy.ru - альтернативная энергетика. [электронный ресурс]. – Режим доступа: http://cyberenergy.ru/vodorodnaya/ (заглавие с экрана)
- Канарёв Ф.М. Начало физхимии микромира. Монография./ Ф.М. Канарёв, 15-е издание. – К.: Краснодар, 2010. – 487 с. [электронный ресурс]. – Режим доступа: http://www.micro-world.su
- Канарёв Ф.М. «ЭЛЕКТРОЛИЗ ВОДЫ» ,2010. [электронный ресурс]. – Режим доступа: http://www.micro-world.su
- Проблемы водородной безопасности. Кириллов И.А., Коробцев С.В. Доклад на Международной конференции «Альтернативные источники энергии для больших городов», Москва, 12-13 октября 2006 г.
- Костин Н. А. Импульсный электролиз /Н. А. Костин, В. С. Кублановский, А. В. Заблудовский, — К.: Наук. думка, 1989.— 168 с. [электронный ресурс]. – Режим доступа: http://mirknig.com/knigi/nauka_ucheba/1181445685-impulsnyy-elektroliz.html
- Князевский Б. А. Охрана труда в электроустановках /Б. А. Князевский, Т. П. Марусова, Н. А. Чекалнн, Н. В. Шипунов; под ред. Б. А. Князевского,— 3-е нзд. — М.: Энергоатомиздат, 1983.— 336 с.
