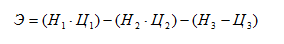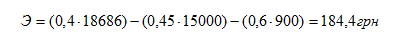Abstract
Content
- Introduction
- 1. State of the art
- 2. Investigation of the effect of phase mixing in steelmaking units in the distribution of elements between metal and slag
- Conclusion
- References
Introduction
With all the rising prices of energy and materials is becoming urgent the problem of saving and rational use of alloying materials and auxiliary materials in the production of steel. The study of processes occurring during the smelting and processing of steel allows you to find ways to conserve materials.
1. State of the art
In the process of finding the oxidized metal and slag in contact (eg in contact with the ladle furnace slag in the production of metal from the furnace) is the recovery of manganese and phosphorus. This process is explained superequilibrium silicon content in a given period of time Thus, while the silicon content is not reduced to the equilibrium values ??in the reduction of manganese and phosphorus from the slag. After the burning process of transition of silicon manganese, and phosphorus is drawn - is their reduction to equilibrium values. For example at LLC "Electrostal" Kurakhovo some batches were obtained coefficients of absorption of manganese excess of 100%: 100.6%, 123.6%, 110.3%.
2. Investigation of the effect of phase mixing in steelmaking units in the distribution of elements between metal and slag
To study the behavior of manganese during the finding of metal and slag in contact on the issue of a furnace held simulation of steelmaking 10G2S through a system of "oracle". To begin with, and obtained a semi-slag chemical compositions are given in Table 1 and Table 2 respectively.
| O | C | Mn | Si | S | P | Cr | Ni | Cu |
|---|---|---|---|---|---|---|---|---|
| 0.15 | 0.37 | 0.23 | 0.0003 | 0.23 | 0.0055 | 0.16 | 0.23 | 0.24 |
| SiO2 | FeO | Al2O3 | CaO | MgO | MnO | P2O5 |
|---|---|---|---|---|---|---|
| 22.2 | 25 | 8 | 28 | 6 | 11.067 | 0.5 |
Upon receipt of the metal and slag with a certain chemical composition in the "kinetics" draw graphical study of the processes occurring after the release of the metal in the bucket for two cases: with a hit in the ladle furnace slag in the production of slag and the cut-off. The processing time will take 40 minutes to become, a lot of the reaction zone of the metal will take 0.5 and 0.08 to the slag thus simulating the metal has a higher rate than the mixing of the slag. The first case - to become 10G2S, in the process of production furnace slag, a large number of falls into the ladle with the metal. Table 3 lists the materials that are used to achieve a given chemical composition of steel.
| Time | Material | Weight, t |
|---|---|---|
| 0 | Metal (распл) | 100 |
| 1 | Al (11) | 0,2 |
| 1 | Coke | 0,1 |
| 2 | СМн17 | 1,150 |
| 3 | ФС65 | 1,2 |
| 5 | Slag (распл) | 6 |
| 16 | Lime | 1 |
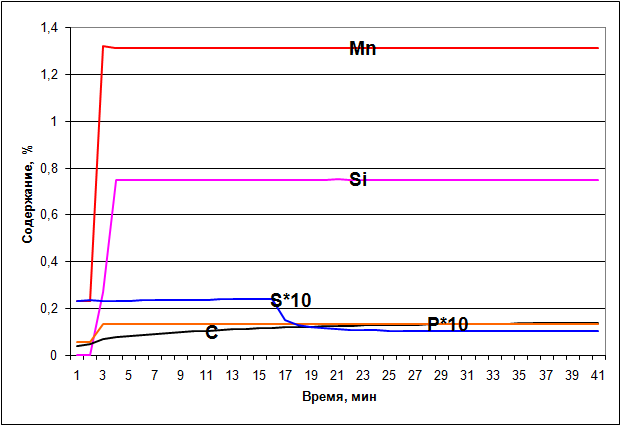
Figure 1 shows the obtained during the simulation depending on describing the behavior of manganese and other elements.
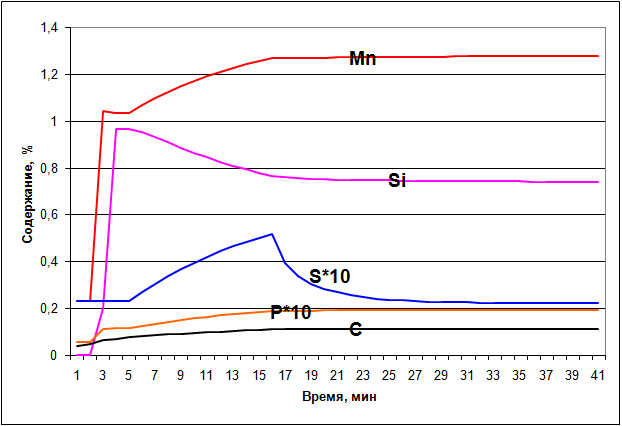
Figure 1 - The behavior of the elements in contact with the slag in the ladle
As can be seen from the graph after the slag is a 5 minute falls into the bucket begins recovery manganese from the slag and this happens until the silicon content reaches the equilibrium values. Next, consider the processes occurring in the case when the issue is preventing the ingress of slag in the ladle. Table 3 lists the materials that are used to achieve a given chemical composition of steel.