Table of contents
- Introduction
- 1. Theme urgency
- 2. Goal, tasks and current results
- 3. Scientific novelty of the system being developed
- 4. Researching results
- 5. Conclusion
- References
Introduction
In modern conditions, more than 400 million tons particles of ash, soot, dust and various kinds of waste and building materials fall into the atmosphere. In addition to the above substances, other, more toxic substances are also emitted into the atmosphere: vapors of mineral acids (sulfuric, chromic, etc.), organic solvents, etc. At present, there are more than 500 harmful substances that pollute the atmosphere [1].
According to the report on the state of the environment of the Donetsk city for 2006-2007, the atmosphere of the city is polluted by the following harmful substances: 916 tons of light organic compounds, 19.2 tons of persistent organic pollutants, 108 tons of cyanides, 130 tons of metals and their compounds, and 15 tons of freons [2]. According to the World Health Organization (WHO) for 2014, approximately 3.7 million people die every year due to air pollution. The total number of deaths associated with exposure to polluted air, both indoor and outdoor, reaches 7 million per year. According to the International Agency for the Study of Cancer, WHO, air pollution is the main cause of oncological diseases [3].
Road transport is the most aggressive in comparison with other modes of transport in relation to the environment. It is a powerful source of its chemical, noise and mechanical pollution. It should be emphasized that with the increase in the car fleet the level of harmful impact of vehicles on the environment is intensively increasing. So, if in the early 70s, environmental scientists determined the proportion of pollutants introduced into the atmosphere by road, an average of 13%, it has now reached 50% and continues to grow. And for cities and industrial centers, the share of vehicles in the total amount of pollution is much higher and reaches 70% or more, which creates a serious environmental problem accompanying urbanization [4].
Theoretically, it is assumed that with complete combustion of fuel as a result of the interaction of carbon and hydrogen (included in the fuel) with oxygen of the air, carbon dioxide and water vapors are formed. In practice, due to the physical-mechanical processes in the engine cylinders, the actual composition of the exhaust gases is very complex and includes more than 200 components, a significant part of which is toxic [5].
The most significant effect on living organisms is produced by carbon monoxide (CO), which belongs to hazard classes 2, 3, according to the UN classification. Carbon monoxide actively binds to hemoglobin, forming carboxyhemoglobin, and blocks the transfer of oxygen to tissue cells, which leads to a significant lack of oxygen in the organs and tissues [6].
Thus, it is obvious that there is a need to control the content of carbon monoxide in the exhaust gases of road transport.
1. Theme urgency
At the moment, there are many systems and devices for detecting carbon monoxide in the samples. Such systems are based on various methods, for example, such as: electrochemical, optical, chromatographic, and ionization. However, such systems are mainly laboratory installations or instruments that work directly with a specific sample that enters the measuring channel either automatically or forcedly [7].
The master's thesis is devoted to the development of a system that will be able to measure carbon monoxide concentrations in motor vehicle emissions automatically, without operator involvement, directly in the flow of moving vehicles. Also, various perturbations and external factors, such as wind presence, turbulent diffusion, etc., will be taken into account. The peculiarity of the developed system is that measurements will take place in an open measuring channel, which eliminates the need for preliminary sampling.
2. Goal, tasks and current results
The goal of the master's thesis is to develop and justify a system for the continuous monitoring of carbon monoxide concentration in the exhaust gases of road transport (hereinafter referred to as SCME), which will be located directly on the carriageway, and measure the concentration of carbon monoxide in real time, in the flow of moving vehicles, and also register license plates of cars, the content of carbon monoxide in the exhaust gases in which exceeds the maximum permissible concentration, regulated in the state standards.
To achieve this goal, the following main tasks have been accomplished:
- methods for measuring the concentration of gas components in atmospheric air have been analyzed, a method has been chosen that ensures the required speed and parameters;
- a structural diagram of the device has been developed that implements the accepted measurement method;
- a mathematical model of the gas environment and a measuring system has been developed with the purpose of evaluating its metrological characteristics.
As a result of the solution of the above tasks, a model of a measuring system capable of measuring carbon monoxide concentration from 0 to 1.7% by volume was synthesized, while the measurement error does not exceed 5%.
3. Scientific novelty of the system being developed
Unlike stationary laboratory devices and devices that need sampling, the system being developed will measure the concentration directly in the middle of the carriageway.This is due to the fact that the cuvette, in which measurements usually take place, will not be present in this device.
The role of the cuvette performs a section of the road on which a cloud of exhaust gas will be located for a short period of time, in which the concentration of carbon monoxide will be measured, using a certain method. It was decided to synchronize the use of the camera with the measurement of concentration, and to take photographs of license plates of cars, the carbon monoxide content in the exhaust gases exceeding the maximum permissible concentration regulated in state standards.
Thus, it can be concluded that this researching would be particularly effective in the CIS countries, where such installations are not widely used. The use of such systems in industrial centers and large megacities would significantly reduce the amount of road transports that emits enormous amounts of harmful substances.
Researching results
Having carried out a detailed analysis of the various optical methods of gas analysis, and guided by the experience accumulated in foreign research works, it was decided that it would be most expedient to develop a measuring system based on the optical-absorption method, which is based on the Beer-Lambert law [8]. The principle of the law is shown in figure 1.
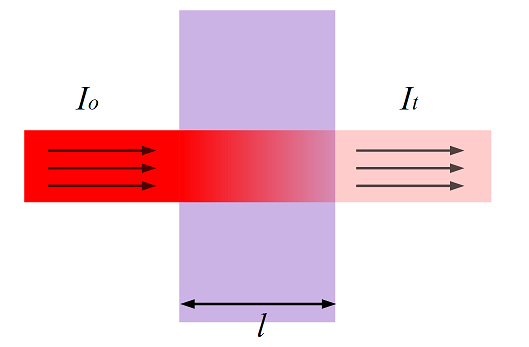
Figure 1 – Measurement principle (Beer-Lambert law) [9]
In accordance with [10], it should be assumed that the exhaust gases propagate in space like a Gaussian distribution. Therefore, in accordance with [11], the differential equation submitted as
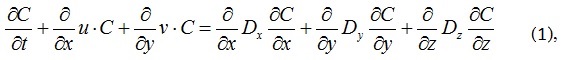
where u, v – wind speeds along the x and y axes, respectively, m/s; Di – diffusion coefficients along the x, y, z axes; C is the concentration of the substance, vol. %.
The solution of this equation in accordance with [11] is the following expression
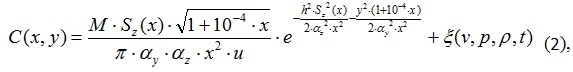
where M is the mass emission of harmful substances, g/s; Sz(x), ay, az – some dispersion factors related to weather conditions; u – speed of the car; x, y are the spatial coordinates; h – height of the exhaust pipe nozzle, m; ξ(v,p,ρ,t) is a function of external influences.
For the convenience of presentation and clarity, a speculative model for the distribution of the exhaust cloud according to the expression (2) was presented (see figure 2).

Figure 2 – Propagation of the exhaust gases cloud in space
(animation: 5 frames, 5 cycles of repetition, 199 KB)
(С1, С2, С3, С4, Сmax – concentration values in some coordinates x, y, z;
h – pipe nozzle height)
Since the concentration of the investigated components in the cloud of exhaust gases is unevenly distributed, and the Beer-Lambert law suggests the opposite [8], it becomes necessary to go over to the integral form of expressing the law to take into account the uneven distribution of concentration:
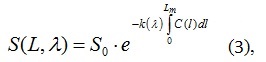
where S(L,λ) is the spectral power density at the output of the system, [VA·sec]; S0 is the power spectral density at the input of the system, [VA·sec].
However, the distribution of the cloud in space presented in the figure does not fully correspond to reality. Obviously, the scattering of a cloud of exhaust gases in space is affected by many different external factors (wind, turbulence in the scattering medium, temperature, pressure, etc.). External influencing factors, as a rule, represent some random process (see figure 3).

Figure 3 – Random process
Real laws were obtained for the distribution of the concentration of harmful substances in the exhaust gas cloud, taking into account external influences (see figure 4).

Figure 4 – Real distribution laws of impurities in different sections
(from above – section C1, from below – C4)
As a result of simulation of the measuring process, an algorithm for generating a calibration signal and a decimal code corresponding to the levels of the given signal was compiled. Each signal level corresponds to some average concentration value measured on the section of the roadway. Having approximated this dependence, the corresponding calibration characteristic of the measuring instrument was obtained and constructed (see figure 5).

Figure 5 – Calibration characteristic
Conclusion
Thus, at the current stage of the research the following results were obtained:
- the chosen method underlying the measuring process is analyzed and justified;
- a speculative model for the distribution of exhaust gases in space is presented;
- a mathematical model for the dispersion of an exhaust cloud is developed, taking into account the influence of external influences;
- the modeling of the measuring process was carried out taking into account the existing developed model, as a result of which the metrological characteristics of the measuring instrument were evaluated.
At the next stage it is supposed to develop a mathematical model of measurements in several cross-sections, as well as the development of a more informative structural diagram, the circuit diagram and the system design.
This master's work is not completed yet. Final completion: May 2018. The full text of the work and materials on the topic can be obtained from the author or his head after this date.
References
- Грандарс [Электронный ресурс]: Загрязнения окружающей среды – электронные данные, – режим доступа http://www.grandars.ru/shkola/bezopasnost-zhiznedeyatelnosti/zagryazneniya-okruzhayushchey-sredy.html – дата доступа: сентябрь 2017.
- Электронный архив Донецкого национального технического университета (г. Донецк) [Электронный ресурс]. – режим доступа: http://ea.donntu.ru:8080/jspui/handle/123456789/17066 – дата доступа: сентябрь 2017.
- Загрязнение атмосферы Земли – Википедия, свободная энциклопедия [Электронный ресурс] – режим доступа: https://ru.wikipedia.org/wiki/Загрязнение_атмосферы_Земли – дата доступа: сентябрь 2017.
- Транспорт и окружающая среда: Учебник/М.М. Болбас, Е.Л. Савич, Г.М. Кухаренок, Р.Я. Пармон и др. – Мн.: Технопринт, 2003. – 262 с.
- Устройство автомобиля [Электронный ресурс]: Загрязнение автотранспортом окружающей среды – электронные данные, – режим доступа http://ustroistvo-avtomobilya.ru/sistemy-snizheniya-toksichnosti/zagryaznenie-avtotransportom-okruzhayushhej-sredy/ – дата доступа: сентябрь 2017.
- Отравление угарным газом – Википедия, свободная энциклопедия [Электронный ресурс] – режим доступа – https://ru.wikipedia.org/wiki/Отравление_угарным_газом – дата доступа: сентябрь 2017.
- Горелик Д.О. Мониторинг загрязнения атмосферы и источников выбросов. Аэроаналитические измерения. 1992. – 432 с.
- Закон Бугера-Ламберта-Бера – Википедия, свободная энциклопедия [Электронный ресурс] – режим доступа https://ru.wikipedia.org/wiki/Закон_Бугера_-_Ламберта_-_Бера – дата доступа: сентябрь 2017.
- Spectrophotometry [Электронный ресурс]. – Режим доступа: https://chem.libretexts.org/Core/Physical_and_Theoretical_Chemistry/Kinetics/Reaction_Rates/Experimental_Determination_of_Kinetcs/Spectrophotometry – дата доступа – сентябрь 2017.
- Р. А. Васькін, В. О. Соляник , І. В. Васькіна – Моделювання розподілу концентрації викидів від автотранспорту у просторі, 2015.
- Модели рассеивания примеси – Википедия, свободная энциклопедия [Электронный ресурс]: – режим доступа https://ru.wikipedia.org/wiki/Модели_рассеивания_примеси – дата доступа: январь 2017.
