ABSTRACT
Content of the abstract
- Introduction
- 1. Relevance of the topic
- 2. Objective
- 3. Setting research objectives
- 4. Analysis of the object of control. Review of existing methods for measuring benzo (a) pyrene
- 4.1. Air pollution in Donetsk
- 4.2. Methods for measuring vehicle emission parameters
- 5. Designing a device for measuring the concentration of benzo(a)pyrene
- 5.1. Development of the block diagram of the device for measuring the concentration of benz(a)pyrene
- 5.2. Transmitter Design
- Conclusion
- References
Introduction
Assessment of air pollution in the city of Donetsk was carried out according to the data of the Donetsk Hydrometeorological Center for average daily concentrations of harmful substances at the control posts [1].
The general level of air pollution in Donetsk is above the average for Donbass and is estimated by experts as extremely high.
Experts determine in the air of Donetsk more than two dozen different harmful substances. Most among them: dust, soot, sulfur dioxide, hydrogen oxide, formaldehyde, hydrocarbons, etc.
One of the main air pollutants of Donetsk and Donetsk region, according to the Department of Environmental Control of the Ministry of Environmental Protection is – transport.
The high level of pollution in the city of Donetsk requires effective measures aimed at reducing the toxicity of exhaust gases from road transport.
At this time, monitoring the concentration of benzo(a)pyrene emissions in exhaust gases is extremely rare, since the detection of benzo(a)pyrene is time consuming and at low concentrations, known methods for detecting results do not give. It should be remembered that the control of this parameter – benz(a)pyrene is extremely important for our region, because of this component, the incidence of cancer in our region is extremely high. Therefore, more frequent control is needed in the residential area of the city.
1. Relevance of the topic
Air pollution is one of the most serious environmental problems of many industrial cities. The effect of air pollution on human health is manifested through a reduction in life expectancy, an increase in the number of premature deaths, an increase in morbidity and a negative impact on the development of children. Therefore, measurement of exhaust gases is an important part of the concept of environmental protection.
The purpose of the work is to develop a block diagram of the instrument for monitoring the concentration of benzo(a)pyrene in emissions from road transport.
3. Setting research objectives
To achieve this goal it is necessary to study and solve the following tasks:
- Analyze the existing methods for detecting the concentration of benzo(a)pyrene in emissions from road transport, choose a method that will allow more accurately detect benzo(a)pyrene in a gas cloud.
- Develop a block diagram of the instrument for measuring the concentration of benz(a)pyrene in emissions from road transport.
- Design a measuring instrument for measuring the concentration of benzo(a)pyrene in emissions from motor vehicles.
4. Analysis of the object of control. Review of existing methods for measuring benzo(a)pyrene
4.1. Air pollution in Donetsk
Car – the most massive and affordable mode of transport, carrying out local and international passenger transportation and serving all sectors of the economy. The number of cars in the world is constantly growing. To date, the number of cars is more than 700 million units.
Unfortunately, road transport is one of the main sources of air pollution in large cities and plays a negative role in the formation of sanitary conditions, both on highways and in residential areas. Almost all cars are equipped with internal combustion engines. On average, each car has about 3 kg of emissions of harmful substances daily. During the combustion of motor fuel in the exhaust gases of vehicles over 300 different substances are detected. Of these, the most common – lead, oxides of nitrogen, sulfur and carbon, hydrocarbons, soot, benzopyrene, various types of dust.
Exhaust gases – the main source of toxic substances of the internal combustion engine. It is a heterogeneous mixture of various gaseous substances with various chemical and physical properties, consisting of products of complete and incomplete combustion of fuel, excess air, aerosols and various trace impurities (both gaseous and in the form of liquid and solid particles) coming from the engine cylinders to its outlet the system. In their composition, they contain about 300 substances, most of which are toxic.
The main standardized toxic components of engine exhaust gases are oxides of carbon, nitrogen and hydrocarbons. In addition, saturated and unsaturated hydrocarbons, aldehydes, carcinogens, soot and other components enter the atmosphere with exhaust gases. When the engine is running on leaded gasoline, there is lead in the exhaust gas composition, and diesel engines running on engines – soot.
Hydrocarbons (CnHm – ethane, methane, ethylene, benzene, propane, acetylene, etc.)
Hydrocarbons – Organic compounds whose molecules are built only from carbon and hydrogen atoms are toxic substances. In the exhaust gases contains more than 200 different hydrocarbons, which are divided into aliphatic (open or closed chain) and containing benzene or aromatic rings. Aromatic hydrocarbons contain one or more cycles of 6 carbon atoms in a molecule, connected by simple or double bonds (benzene, naphthalene, anthracene, etc.). Have a pleasant smell.
The presence of hydrocarbons in the exhaust gases of engines is explained by the fact that the mixture in the combustion chamber is heterogeneous, therefore, the walls, in the over-enriched zones, extinguish the flame and break the chain reactions (see figure 4.1.1).
Incompletely burnt hydrocarbons emitted with exhaust gases and a mixture of several hundred chemical compounds have an unpleasant odor. Hydrocarbons are the cause of many chronic diseases.
Gasoline vapors, which are hydrocarbons, are also toxic.
The permissible daily average gasoline vapor concentration is 1.5 mg/m^3. The content of hydrocarbons in the exhaust gases increases with throttling, when the engine is running at forced idling (PHC, for example, when braking the engine.).
When the engine is operated at the specified modes, the process of mixture formation deteriorates (mixing of the air-fuel charge), the burning rate decreases, the ignition deteriorates and, as a result, – there are his frequent omissions.
The release of hydrocarbon is caused by incomplete combustion near the cold walls, if there are places with a strong local air deficit, insufficient fuel atomization, with poor turbulence of the air charge and low temperatures (for example, idling) until the end of combustion.

Figure 4.1.1 – CH Formation in exhaust gases
1 – piston; 2 – sleeve; 3 – wall layers of the mixture
Hydrocarbons are formed in over-enriched zones where oxygen is restricted, as well as near the relatively cold walls of the combustion chamber. They play an active role in the formation of biologically active substances that cause irritation of the eyes, throat, nose and their disease, and damage the plant and animal world.
Hydrocarbon compounds have a narcotic effect on the central nervous system, can cause chronic diseases, and some aromatic hydrocarbons have toxic properties.
Hydrocarbons (olefins) and nitrogen oxides under certain meteorological conditions actively contribute to the formation of smog.
Smog – poisonous mist formed in the lower layer of the atmosphere polluted with harmful substances from industrial enterprises, exhaust gases from motor vehicles and heat-producing installations under adverse weather conditions.
It is an aerosol consisting of smoke, fog, dust, soot particles, liquid droplets (in a humid atmosphere). It occurs in the atmosphere of industrial cities under certain meteorological conditions.
The harmful gases entering the atmosphere react with each other and form new ones, including toxic compounds. At the same time, photosynthesis, oxidation, reduction, polymerization, condensation, catalysis, etc. take place in the atmosphere.
As a result of complex photochemical processes stimulated by ultraviolet radiation from the sun, photooxidants (oxidizers) are formed from nitrogen oxides, hydrocarbons, aldehydes and other substances.
Низкие концентрации окиси азота, могут создать большое количество атомарного кислорода, который в свою очередь образует озон и вновь реагирует с веществами, загрязняющими атмосферный воздух. Наличие в атмосфере формальдегида, высших альдегидов и других углеводородных соединений также способствует вместе с озоном образованию новых перекисных соединений.
Dissociation products interact with olefins to form toxic nitro-peroxide compounds. When their concentration is more than 0.2 mg/m^3, condensation of water vapor occurs in the form of the smallest droplets of mist with toxic properties. Their number depends on the season of the year, time of day and other factors. In hot, dry weather, smog is observed as a yellow veil (color imparts NO2 nitrogen dioxide present in the air – droplets of yellow liquid).
Smog causes irritation of the mucous membranes, especially the eyes, can cause headaches, swelling, hemorrhages, complications of respiratory diseases. Visibility on roads deteriorates, thereby increasing the number of traffic accidents.
The danger of smog for human life is great. For example, the London smog of 1952 is called a catastrophe, since about 4 thousand people died from smog in 4 days. The presence in the atmosphere of chlorine, nitrogen, sulfur compounds and water droplets contributes to the formation of strong toxic compounds and acid vapors, which has a detrimental effect on plants and structures, especially historical monuments built of limestone.
The nature of smog is different. For example, in New York, the formation of smog is facilitated by the reaction of fluoride and chloride compounds with water droplets; in London – the presence of sulfuric and sulfurous acid vapors; in Los–Angeles (Californian or photochemical smog) – the presence in the atmosphere of nitrogen oxides, hydrocarbons; in Japan – the presence in the atmosphere of soot particles and dust.
Donbass – this is a large industrial region, which has several thousand large industrial enterprises, industrial – industrial associations and enterprises of the fuel – energy complex, mining, metallurgical, chemical industry, heavy engineering, construction industry, as well as the agro-industrial complex. Donbass provides most of the industrial production, and in the most environmentally hazardous industries.
High concentration of industrial and agricultural production, transport infrastructure, combined with high population density, created an extremely high anthropogenic and anthropogenic load on the biosphere – the highest in Ukraine and Europe.[6]
Donetsk region, industrial sources of air pollution are depicted by red dots in figure 4.1.2.

Figure 4.1.2 – Map of Donetsk region
Leadership Makeevka (city–satellite of Donetsk) in the ranking of the most environmentally unfriendly cities, an observatory specialist explains by a sharp increase in the level of benzo(a)pyrene – substances of the first class of danger. Its atmosphere in the city is 4.6 times the permissible level. Perhaps this is due to increased emissions of enterprises, perhaps unfortunate weather conditions. Because of the weak wind and reduced dispersibility, benzo(a)pyrene droplets accumulated in the atmosphere.
The head of the ecology department of the Makeevsky city council claims that a high rate of pollution, in the first place, is provoked by a large number of industrial enterprises.
We have one metallurgical plant, two coke–chemical, one pipe–casting.
In addition, the coal industry, primarily mines, which have not been completely closed, causes great harm to the environment.
, – says the official.
According to him, there are heaps near the mines.
Some of them are smoking.
As a result, dust and dirt spread throughout the city.
4.2. Methods for measuring emissions of vehicles
Methodical instructions establish a method for the quantitative chemical analysis of air media (air of the working area and atmospheric air of populated areas) for determining benz(a)pyrene in them by high performance liquid chromatography with fluorimetric detection. [5, 8] The range of measured concentrations: – in the atmospheric air of populated areas is 0.0005 – 10 µg/m^3 when sampling 5 m^3 samples; – in the air of the working area is 0.02 – 5000 µg/m^3 when sampling a volume of 150 – 300 dm^3.
Benz(a)pyrene (3.4–benzpyrene) belongs to the class of polynuclear aromatic hydrocarbons, the molar mass of 252.32 g/mol. The structural formula of the molecule is presented in figure 4.2.1.

Figure 4.2.1 – The structural formula of the molecule benzo (a) pyrene
The substance is a product of incomplete combustion (pyrolysis) of organic compounds, is present in the products of processing of coal, oil (heavy fractions), and is also formed in large quantities in the production of aluminum. Benz(a)pyrene has carcinogenic activity. This harmful substance can penetrate the human body in several ways: through the upper respiratory tract, skin and mucous membranes, as well as with saliva through the digestive organs. The most dangerous is the penetration through the upper respiratory tract, as in this case, the absorption of toxins is quite intense, and benzo(a)pyrene through the lungs immediately enter the blood of benzo(a)pyrene is practically not excreted from the body throughout life. With its concentration equal to an average of 0.1 µg/m^3 of air, the total content in the human body over 70 years of life, when inhaled with inhaled air, reaches 16 mg and can cause cancer. In the atmosphere, predominantly benzo(a)pyrene is adsorbed on suspended particles.[10]
Benz(a)pyrene is a hazard class 1 substance. The average daily maximum permissible concentration of benzo(a)pyrene in the air of populated areas is 0.001 µg/m^3, the maximum permissible concentration of pollutants in the ambient air of populated areas, the maximum single maximum permissible concentration has not been established. The maximum permissible concentration in the air of the working zone is set at 0.15 µg/m^3.
The measurement method is based on capturing benzo(a)pyrene on an aerosol filter, extracting it with hexane, concentrating the extract, chromatographically separating it, registering a signal using a fluorescent detector, identifying the peak of benzo(a)pyrene on the chromatogram by retention time and calculating the mass concentration of benzo(a)pyrene.[11]
The chromatographic column should be filled with a reversed phase sorbent and, under the conditions of the analysis, it should have an efficiency of at least 5000 theoretical plates for the peak of benzo(a)pyrene. The minimum detectable concentration of benzo(a)pyrene in solution (at a signal-to-noise ratio of three) should be no more than 0.002 ?g/cm^3. The column is equipped with a prefabricated column filled with a similarly reversed – phase sorbent.
Solutions of benzo(a)pyrene should be stored in a refrigerator in hermetically sealed containers. Since benz(a)pyrene has a pronounced carcinogenic activity, it is necessary to avoid contact of the skin with its solutions and after work, as well as if the solution gets on the skin or on the table, it is necessary to wash the contamination with running water with detergent, and then treat the skin area and contaminated surfaces with ethyl alcohol.
In the process of carrying out this work, the author came to the conclusion that the most suitable for operational monitoring of the atmospheric air for the presence of benzo(a)pyrene is the method of dispersionless spectrometry.
The method is based on selective absorption in a narrow wavelength range (in the absorption band) by a substance of optical radiation.
Substance is characterized: frequency–dependent absorption coefficient ε(λ). For simple substances, the absorption spectrum has the character of a hausian, for complex substances it is a set of simple components, taken in certain proportions. As a result of this mixing of components, the spectrum is obtained of a complex form, but all the spectra are individual in appearance, the substance can be identified in the form of the spectrum.
The identification process in the figure. 4.2.2:

Figure 4.2.2 – Identification process
Substance having a molar absorption coefficient ε(λ) is a part of a two-component solution, where there is a solvent and analytes. The analyte can be located in the cuvette of the measuring device or directly the analysis can be carried out in the medium (water medium, soil, air) inside technological installations.
The analyzed medium is irradiated with a flux of radiation Фi. The radiation spectrum should contain components in the absorption band of the analyte.
The interaction of the analyte with the radiation flux is described in the spectral region:

Where C – concentration, l – optical path length in matter (this parameter must be const).
Optical density D is a function of two variables. ε and C.
The radiation flux passing through the layer of matter is partially absorbed. This can be described as follows:
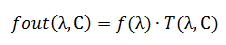
The output stream contains information about the component being analyzed. Quantitatively, this change can be defined as follows:
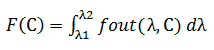
The conversion must be completed by converting an optical signal, which is a radiation containing information about the component being analyzed, into an electrical signal that will contain the transmitted information.

If a photodiode is used as a photodetector, the electrical signal will be represented as a photocurrent of this diode, the magnitude of which carries information about the component being analyzed.
5. Designing a device for measuring the concentration of benzo(a)pyrene
5.1. Development of the block diagram of the device for measuring the concentration of benz(a)pyrene
To implement the method of non-dispersive ultraviolet spectrometry, the device requires: a radiation source, the output signal of which will irradiate the working cell, and the output will have a signal with a concentration of the test substance in it. Working cell, which will undergo analysis and measurement of the concentration of benzo(a)pyrene.
The exhaust gases to be analyzed will be passed through a liquid reagent, in this case hydrogen tetrachloride, mixed using a sprayer in order to achieve a uniform consistency. A photodetector is also needed, which will receive the output signal after irradiating the cell with the concentration component in the signal. The output signal of the photoreceiver will be tens of ?A, therefore we will need a current-to-voltage converter.
In order to supply a better signal to subsequent devices, we need to increase the voltage with the help of a normalizing amplifier to the required level. Since quite a lot of measurements will be made, in order to save intermediate results, we introduce a sampling device–storage.
Research of exhaust gases does not boil down to determining only the parameter of benzo(a)pyrene concentration, other tasks can be solved, in particular: as part of the exhaust gases, it is possible to determine other components included in the exhaust gases of the car.
In order to automate measurement processes, as well as sample preparation, a microprocessor system should be introduced into the device. This system should operate in real time, the following should be provided: control of the multiplexer for the choice of measuring channels connected to the ADC; control device UVH; management of the launch of the ADC; management of switching processes of ultraviolet radiation sources; management of signaling in case of abnormal violations of environmental parameters. To solve all these problems, as well as apart from them, the microprocessor system must solve the problem of ensuring the required metrological level of the measuring system. That is, to periodically eliminate accumulated errors, due to aging of the components of the measuring instrument, to eliminate in addition incoming errors from external disturbances: changes in ambient temperature, humidity, pressure, voltage of power sources, degradation of optical components.
The microprocessor system should also deal with the displayed data. The microprocessor system must also set the measurement result; for a microprocessor system with analog information sources, the multiplexer of analog signals, an analogue and digital converter, should be introduced into the system.
The microprocessor system should also deal with the displayed data. Taking into account all the above, the block diagram of the instrument for measuring the concentration of benzo(a)pyrene should have the following form shown in the figure 5.1.1.
The block diagram visually shows the main functional units of the device, their purpose and their participation in the general process of measuring the concentration of benzo(a)pyrene in the exhaust of motor vehicles, is a combination of the object links and the connections between them.
On the structural scheme, the following notation is used:
- RSCD – radiation source control device;
- RS – radiation source;
- WD – working ditch;
- PD – photodiode;
- MT – measuring transducer;
- CVC – current to voltage converter;
- НA – normalizing amplifier;
- ASM – Analog signal multiplexer;
- SS – storage sampler;
- АDC – analog–digital converter;
- MS – microprocessor system;
- IDD – information display device;
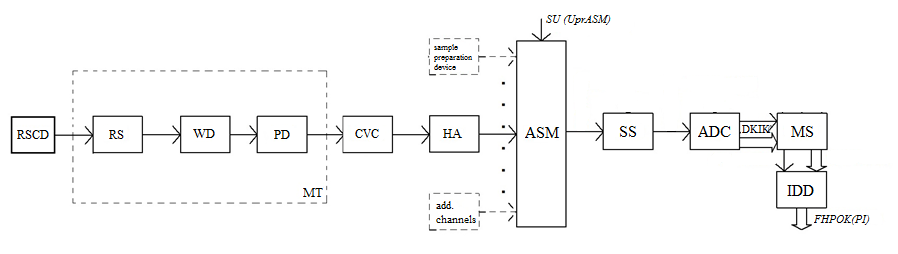
Figure 5.1.1 – Block diagram of the instrument for measuring the concentration of benzo(a)pyrene
5.2. Transmitter Design
The implementation of the chosen method of measuring the conversion of benzo(a)pyrene to an electrical signal requires, as main components, an ultraviolet radiation source with a wavelength corresponding to the middle of the absorption spectrum benz(a)pyrene and a photodetector with a spectral characteristic covering the absorption spectrum and the spectrum of the analytical signal.
The absorption spectrum of benzo(a)pyrene is shown in figure. 5.2.1. The spectrum lies in the range from 240 nm to 340 nm.

Figure 5.2.1 – Benz(a)pyrene absorption spectrum
Accepted as the emitter of the light emitting diode ultraviolet range T9F28C with parameters: λisl – radiation wavelength, λisl = 280 nm; λi – spectrum width, λi = 15 nm ; Fi = 600 mkW, ΔF – radiation angle, ΔF – 15°.Forward voltage nominal – 5,7 V, Direct current rated 10 mA. The spectrum of a light-emitting diode covers the most significant part of the absorption spectrum of benz(a)pyrene, in the range from 265 nm to 295 nm.
As a photodetector we use a photodiode UVD–20 having parameters: S(λmax) – monochromatic photodetector sensitivity at a frequency of maximum spectral sensitivity of 0.25 A/W; λmax – 380 nm, level band 0,1 from 200 – 540 nm; dark current – 0,7 on; sensitive window size – a•b = 1•1 mm. The spectral sensitivity of the photodetector is shown in the figure 5.2.2.

Figure 5.2.2 – Spectral sensitivity of the photodetector
The emission spectrum of the LED, which contains information about the absorption spectrum in the range from 265 nm to 295 nm, is included in the sensitivity spectrum of the photodetector and can be converted into an electrical signal.
The structure of the optical system presented in Fig.5.2.3 includes: radiation source (AI) – light emitting diode, 2 one lens Obl and Ob2 lenses, working cell, photodiode (FD) – photo detector (OP1). The materials of the cuvette lenses should be optically transparent in the range of the analytical signal. As a material for the optical component, fused silica is used, transparent to the wavelength λ=190 nm.
The optical signal propagation through the optical scheme requires taking into account the loss of optical radiation in the reflection of a part of the stream during the passage of a part of the air flow - optical component, as well as taking into account radiation losses when entering radiation into lenses and into a photodetector. Perform a sequential calculation of the parameters of optical signals on the way from the light-emitting diode to the photodetector.
The numbering of flows is shown in the figure. 5.2.3 – optical meter circuit.

Figure 5.2.3 – Optical circuit meter
As a flow F0 the nominal value of the light emitting diode flux is taken;
Estimation of the amount of flow entering the pupil About1 and its output value – F12
We will estimate the flow value according to the figure. 5.2.4 – the passage of radiation through a single lens lens.

Figure 5.2.4 – The passage of radiation through a single lens lens
In the work done:
- The analysis of existing methods for detecting the concentration of benz(a)pyrene in emissions of road transport was carried out. A method has been chosen that will allow more accurate detection of benzo(a)pyrene in the gas cloud;
- A block diagram of an instrument for measuring the concentration of benzo(a)pyrene in emissions from motor vehicles has been developed;
- Developing a circuit implementation of the instrument for measuring the concentration of benzo(a)pyrene in emissions from road transport;
When writing this essay, the qualification work of the master is not yet completed. Date of final completion: June 2019. Full text and materials on the topic of work can be obtained from the author or supervisor after that date.
References
- Аверин Г. Доклад о состоянии окружающей среды в Донецкой области / Аверин Г., Кишкань Р., Аверин Д., Звягинцева А. – Донецк, 2008. – 112 с.
- Марчук Г.И. Приоритеты глобальной экологии / Марчук Г.И., Кондратьев К.Я. – М.: Наука, 1992. – 263 с.
- Методика расчётов выбросов автотранспорта для проведения сводных расчётов загрязнения атмосферы городов. – СПб., 1999. – 16 с.
- Родин В.В. Теория и расчёт измерительных преобразователей и приборов: Методические указания к выполнению лабораторных работ. – Саранск: Изд-во
Референт
, 2007. – 52 с - Канцыпко Е.В. Причины загрязнения атмосферного воздуха в Донбассе. Реферат по дисциплине
Основы экологии
. – Донецк: ДонНТУ, 2008. – 47 с. - Гусев В.Г. Электроника. Издание второе, переработанное и дополненное. / Гусев В.Г., Гусев Ю.М. – М.:
Высшая школа
, 1991. – 624 с. - Казицина Л.А. Применение УФ –, ИК –, ЯМР – и МАСС – спектроскопии в органической химии. Издание второе, переработанное и дополненное. / Казицина Л.А., Куплетская Н.Б. – М.: Моск. ун-та, 1979. – 240 с.
- Рабинович В.А. Краткий химический справочник. Издание второе, переработанное и дополненное. / Рабинович В.А., Хавин З.Я. – М.:
Химия
, 1978. – 392 с. - Кораблёва А.И. Научно-практические аспекты охраны воздушной среды. Учеб.пособие. / Кораблёва А.И., Чесанов Л.Г., Ветвицкий И.Л., Полищук С.З., Чесанов В.Л., Житченко И.В. – Днепропетровск: Монолит, 2008. – 324 с.
- Другов Ю.С. Пробоподготовка в экологическом анализе. / Другов Ю.С., Родин А.А. – СПб.:
Анатолия
, 2008. – 755 с. - Другов Ю.С. Методы анализа загрязнений воздуха. / Другов Ю.С., Беликов А.Б., Дьякова Г.А., Тульчинский В.М. – М.:
Химия
, 1984. – 384 с. - Ровинский Р.Е. Обзор литературных материалов по оптическим методам определения химического состава и концентраций компонентов в газовых средах. – М., 1995. – 30 с.
