Abstract
Content
- Introduction
- 1. Overview of known technologies for the synthesis gas.
- 2. Description of the proposed flowsheet the synthesis gas.
- Technological scheme of the synthesis gas.
- Conclusion.
- List of sources
Introduction
The first method of production of synthesis gas was gasification of coal, which was implemented back in the 30s of the XIX century in England to produce combustible gases: hydrogen, methane, carbon monoxide. This process is widely used in many countries until the mid-50s of the XX century, and was then replaced by methods based on the use of natural gas and oil. However, due to the reduction of petroleum resources, the value of the gasification process once again began to grow.
Production of synthesis gas is an important element of modern technologies of chemical synthesis. Increased competitiveness of production can be achieved either by increasing the overall level of the economy of resources, and due to the effective implementation of new developments in the direction of synthesis gas. In this work we propose a scheme for producing synthesis gas from natural gas through a combination of steam and oxygen conversions.
1. Overview of known technologies for the synthesis gas.
- In an industrial scale for the production of synthesis gas using three main methods:
- Conversion of natural gas with steam and oxygen.
(Reaction of methane with steam is carried out in the presence of nickel catalysts (Ni-Al2O3) at elevated temperatures (800-900 °C) and pressure, as a raw material instead of methane can be used by any hydrocarbons.) - The oxidation of heavy fuel oil.
- Gasification of coal.
When different methods of synthesis gas produced gas with different ratios of CO/H2. Conversion of natural gas by steam-1:3, oxygen - 1:2, other 1:1. The ratio of CO/H2 is of great importance for the further processing of synthesis gas. For example, for methanol synthesis requires the synthesis gas with a ratio of CO/H2 = 1:2.
2. Description of the proposed flowsheet the synthesis gas.
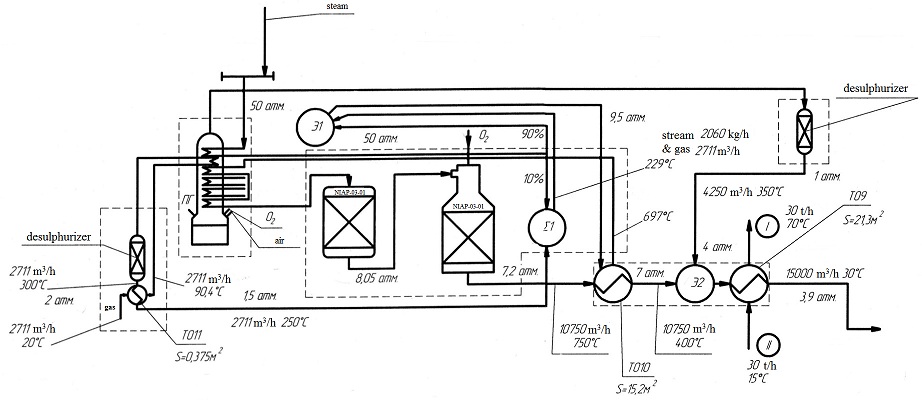
In the section for production of synthesis gas enters the natural gas in an amount of 2711 m3/h and a temperature of 20 °C. Passing through the heat exchanger heat exchanger №11, the gas is heated to a temperature of 90 °C. After that, the gas enters the gas-gas heat exchanger. It is an oxygen conversion of natural gas in the volume of 1289 m3/h from another thread. While in the tube space, which is washed by the products of conversion of natural gas is heated to a temperature of 300 °C and then enters the desulphurizer for removal of sulfur. Passing through the first heat exchanger heat exchanger №11, natural gas gives the heat newly received gas and its temperature drops from 300 °C to 250 °C.
In one of the heat exchangers located in the cell of incomplete combustion, steam is fed to heat, after which the steam is separated into two streams at a ratio of 9:1. The first part of the steam flow (90%) goes directly to the ejector is number 1, as ejecting media. The second part of a couple (10%) is blended in a mixer with natural gas, and the resulting mixture is sent to the same №1 in the ejector, as the ejected medium, in which there is complete mixing natural gas with a residual part of the couple. This mixture with a temperature of 229 °C and a pressure of 9.5 atm. enters the heat exchanger is heat exchanger №10, which is heated to temperatures 697 °C and enters the heat exchanger for further heating. Then the mixture with a high temperature and pressure of 9 atm. be the first reactor NIAP-03-01, where the reaction takes the steam reforming of natural gas by the equation:
Reacted with a mixture of 8.05 atm pressure. directed into the second reactor NIAP-03-01, which further fed oxidizer (O2) for the steam-oxygen conversion of the reaction products, since the steam conversion of natural gas takes place with absorption of heat, and oxygen - with its release. The reaction proceeds according to the equation:
Combining the two methods of conversion, steam and oxygen, can efficiently and produce more complete decomposition of natural gas into its components, so we can talk about reducing the cost of production of synthesis gas.
At the exit from the second reactor, we have a mixture consisting of gases CO and H2, is essentially a synthesis gas, as well as by-products of CO2 and H2O which must be removed before further processing of synthesis gas. Whole volume of gas 10,750 m3/h, with a temperature of 750 °C and a pressure of 7.2 atm. sent to heat exchanger №10 to heat the next portion of the mixture of natural gas and steam.
The combustion products, resulting from the conversion of oxygen gas in the chamber of incomplete combustion, are sent to the desulphurizer. After a clean up of products of incomplete combustion of sulfur at a temperature of 350 °C and a flow rate of 4250 m3/h, mixed with the previously obtained synthesis gas in an ejector №2. The final mixture passes through a heat exchanger №9 cooled process water, to lower the temperature of synthesis gas to a level of 30 °C. Passing a series of heat exchangers, we select a natural heat from the synthesis gas and pass it to once again received a mixture of gas and vapor, thereby increasing the degree of heat.
By passing gas exchangers of the chain, we have a synthesis gas with a pressure of 3.9 atm. and the amount of 15,000 m3/h
The resulting synthesis gas should be sent to the area clean from CO2 and H2O, then it goes to the site for methanol.
Below, in Fig. 1 shows a diagram of the synthesis gas production from natural gas by steam and oxygen conversion.
Figure 1 - Flow diagram of the synthesis gas production.
Conclusion.
In such a way in this paper we propose a process flow diagram of production of synthesis gas and is represented by a specific process flow diagram, allowing to receive 15,000 m3/h of synthesis gas in the processing of 4000 m3/h of natural gas.
List of sources
- Бекиров Т.М. Первичная переработка природных газов / Бекиров Т.М. // Химия – 1987. - 256 с.
- Богомолов А.И., Гайле А.А., Громов В.В. Химия нефти и газа / Богомолов А.И., Гайле А.А., Громов В.В. // Химия – 1995. - 448 с.
- Катализ в Cl-химии / Под ред. Л. Кайма. Л.: Химия, 1987. - 296 с.
- Патент 2052492 РФ. Способ получения синтез-газа и газификатор вертикального типа / С. Р. Исламов, С. Г. Степанов, А. Б. Морозов, О. С. Пивоваров, В. А. Збруев. - Опубл. 20. 01.1996 г. в БИ № 2. - 4 с.
- Шелдон Р. А. Химические продукты на основе синтез-газа: Пер. с англ. М.: Химия. - 1987.
- Синтез-газ
- В.И. Мурин, Н.Н. Кисленко, Ю.В. Сурков и др. Технология переработки природного газа и конденсата. Справочник / В.И. Мурин // Недра - 2002. - 517 с.
- Караханов Э. А. Синтез-газ как альтернатива нефти. / Караханов Э. А. // Соросовский Образовательный Журнал. 1997. № 6. С.69.
- Катализ в С1 – химии. / Под ред. Л. Кайма. Л.: Химия, 1987. 296 с.
- Арутюнов, В. С. Окислительная конверсия природного газа /РАН; Отв. Ред. А. Л. Лапидус. - М.: КРАСАНД, - 2011