Speciality: Chemical Technology of Fuel

SHAKIR SHWAN MOHAMMED
Theme of master's work:
STUDY OF COALS EXTRACTS OF DIFFERENT GENETIC TYPES BY REDUCTIVELY.
Scientific adviser:Professor, Doctor of Science, L.F. Butuzova
Introduction (Motivation)
The development of human society entails a sequence of events associated with changes in both external and internal facts of the world, which are reflected in science. Nature has cherished rich reserves of carbon in the form of coal, oil and natural gas for millions years. Now, these fossil fuels are used by mankind for producing energy and chemical products. At the end of the past and at the beginning of this century, the majority of organic chemical products were made of fossil fuels.
The products of petrochemical synthesis, produced with the help of simpler and less energy intensive methods, began superseding chemicals of coal origin, as oil production increases. However, a rough estimate of the world's reserves of fossil organic material leads to the conclusion that oil and gas will be largely exhausted in the first decades of the XXI century. And coalstocks should be enough for the next several hundred years.
Conclusion is that we need to increase permanently the use of coal in the energy industry and it is confirmed by comparing oil, gas, coal, and the nowadays structure of world consumption [1].The main disadvantages of well-known technologies of chemical processing of coal if we compare them to oil refining and petrochemical technologies are relatively low productivity and harsh conditions for their implementation (high temperature and pressure) [2].
The relevance of the topic
The relevance of the topic is defined by modern general purpose of coal fuel chemistry and petrochemistry, by wide interest in the issues of exhaustion and the sustainable use of natural resources, by the issues of geochemistry and chemistry of in-process fossil fuels. Relevance directly connected with the material of the study, its specificity.
The scientific significance
The scientific significance of the work is to try to improve the current system of utilization and classification of fuels with high sulfur content through the study of extracts of oil and coal. The lack of study of the structure of fossil fuels and ways of transformation of organic material in the coal and petroleum forming requires to obtain new facts concerning the dependence of the coal, oil, and their extracts on the terms of mentioned processes. Particular attention in this work is drawn to comparison of the ways of obtaining fuel from the feed stock. During the study it is possible to deal with a practical comparison of extracts derived from petroleum and coal of different types of restoration. The results will allow to deepen the existing notions about the features of low-sulfur coals and to suggest possible ways of their efficient use.
summary of research and developments
Coal (black and bovey) used as fuel or raw materials for processing, in most cases lies in the land (partially at a depth of many hundreds meters). Only a few bovey coalfields you can find on the surface or directly near the surface layers of the ground.
Still there is no the only opinion about the issue which organic components are the raw material in the coal formation, there is no single pattern of its genetic changes. Mined coal in its composition contains organic materials, it is primarily carbon with oxygen and hydrogen, rarely-with the nitrogen, sulfur and other elements. The main chemical elements that make up the coal are carbon, oxygen and hydrogen [3].
Extraction has been one of the traditional methods of studying of the coal structure for a long time. It was due to the fact that the majority of analytical methods are applicable to liquid or soluble products. Extraction continues to be the main method of study of the molecular structure of coal, so the more that the extraction processes acquire industrial importance and they are reflected in the thermal dissolution of coal, and in dissolution by supercritical solvents [4].
Interest in the extracts repeatedly reinforces that are important for understanding the process of transition coal into the plastic state and the process of caking. Besides in the composition of extracts we can find compounds, which preserve the original structure of vegetable material as a whole or its specific characteristics.
Many researchers studied the extraction of solid fuels with the help of organic solvents. They used the typical organic solvents such as benzene and homological to it, aliphatic alcohols, esters, ketones, etc. Using these solvents with boiling temperatures and atmospheric pressure we can produce extracts with a low yield from coal [5]. It’s known long ago about the influence of extraction the plastic properties of coals.
Extraction with the help of polar aprotic solvent reduces plasticity of coal.The part of loosely coupled material, captured in the micropores of coal is the part of the pitch of low-temperature coal degradation up to (500-550 0C).Despite the fact that the extracts have a high coking properties, residue after extraction has a great influence the process carbon-producing.The structure of extracts was correlated with the structure of original fuel.
One of the most effective solvent for coal is considered to be pyridine, extracting up to 30% of coal at the boiling temperature of solvent and atmospheric pressure. The higher degree of fuel coalification, the lower the yield of pyrites extract.The yield of extracts largely depends on the solvent nature, temperature and duration of extraction.
The method of extraction under pressure has been applied to obtain ash-free raw material for hydrogenation or to obtain diesel fuel since 1925. Initially benzene was used as a solvent, but the drawback of benzene was the low critical temperature of 2900C, and it was replaced by tetralin. 75-85% of tetralin extract is produced from bovey coal [6].
Oil is composed mainly of hydrocarbons, as well as in small amounts of other elements (sulfur, nitrogen, oxygen, etc.). Oil contains 82-87% of carbon and 11-14% of hydrogen. Chemical petroleums are very different and vary from paraffin, which consists mostly of paraffin hydrocarbons to naphthenic or to pyrobitumen, which contain mostly cyclehydrocarbons, there are many intermediate or mixed types. Paraffin petroleums besides naphthenic or pyrobitumen usually contain more gasoline and less sulfur and it is the main raw material for lubricating oils and paraffin producing. Naphthenic types of crude oils generally contain less gasoline, but more sulfur, fuel oil and asphalt [7].
The essence of the method of extraction in the study of highmolecular ends of oil is that in the same solvent, such as acetone or liquid propane at different temperatures can bedissolved substances with different critical temperature of dissolution. Therefore, if we carry out the fractional extraction, i.e. if we select extracts successively at different temperatures, ranging from the lowest to optimum for a given solvent, then we can obtain a number of factions after the distillation of solvent. In each of the selected factions concentrate substances with similar critical temperature of dissolution.
Analyzing the data of these factions we may make a conclusion which of petrochemical product can be obtained at any temperature stage of petroleum refining. It is obvious that such substances will be grouped by more or less similar structure and they will be easier for exploring by other methods.
However extraction by selective solvent is suitable not only for carbon-black, but also for division of oil factions. For example, aromatic hydrocarbons are selectively dissolved in liquid sulfur anhydride, in furfural and nitrobenzene, but they do not dissolve the hydrocarbons of other classes. Resinous substances and polycyclic aromatic hydrocarbons at usual temperatures are well dissolved in phenol, cresol, furfural, nitrobenzene. The first selective extractant was liquid sulfur anhydride which was offered for the kerosene treatment of aromatic hydrocarbons [8].
Crude oil is subjected to the so-called periodic distillation in the vertical cylindrical distiller. Following the distillation of crude oil we may produce the following products:
1. Liquefied hydrocarbon gas, consisting mainly of propane and butane. The number of product depends on that fact how deep the oil was stabilized on the field installation. When oil is with the high content of gas, propane-butane faction is derived from distilling unit not only in the liquid, but also in gaseous state. Liquefied gas can be used as domestic fuel after purification of sulfur compounds. The hydrocarbon gas may also be the feed stock of gas installations.
2. Gasoline faction. It is distillated at (30-1800C) and used as a component of benzene, as feed stock of catalytic reforming plants. Narrow factions of benzine obtained on the plants and on the secondary distillation units, are raw material for producing of individual aromatic hydrocarbons such as benzene, toluene, xylene.
3. Kerosene faction is distillated at (120-3150C), depending on the purpose for which kerosene is used: as a fuel jet aircraft engines, for lighting or as a fuel for tractor carburetor engines. Kerosene fraction needs to be cleaned of sulfur compounds, and it is conducted on special hydrofining plants.
4. Diesel faction is distillated at (180-3500C). Formerly the diesel faction was called the atmospheric gas-oil stock. The faction is used as fuel for diesel engines installed on motor vehicles, tractors, locomotives, ships of marine and ships of river fleet. Diesel faction which is derived from sulfur crude oils, needs to be cleaned-up of sulfur.
5. Mazut is distillated at temperature above (3500C), it is used as boiler fuel, raw materials for the thermal cracking plants. The range of products of vacuum distillation of mazut depends on the option chosen for petroleum refining. There are two schemes of mazut distillation : oil distillation and fuel distillation. When the oil distillation is used we may produce several factions-vacuum distillates, which number is determined by the type of processed petroleum, when the fuel distillation is used we may produce only one faction.
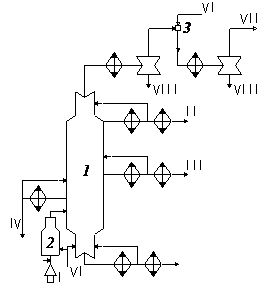
1-vacuum column;2-vacuum furnace; 3–steamejector vacuum pump; I-mazut; II–light vacuum gas-oil stock; III–vacuum gas-oil stock; IV–tinted faction; V–tar; VI–steam; VII–decomposition gas; VIII–condensate (water and petrochemical product) [9].
According to the existent scheme of obtaining oils of the eastern petroleum, the plants of primary distillation should receive three vacuum distillates: light faction at (300-400 С), middle faction at (400-4500C), heavy faction at (450-5000C). Each of distillates is heated and cleaned, then purified products are mixed in different proportions and according to this there is a variety of oils.
6. Tar is residue of petroleum after distillation, it’s distillated at a temperature above (5000C). It’s - highly viscous product, solidifying at(300-4000C), it is used as a raw material for thermal cracking plant, for coking, for the production of bitumen and highly viscous oils. Coal and petroleum have common origin and so they have a similar products of obtaining. Products derived after extraction also can be shared and distinct from each other [10].
Conclusions and future research
Although nowadays petroleum is the main source of organic raw materials, the limitations of the world total supplies and permanent growth of the cost of mining due to involvement of more difficult fields stimulate the development of new processes of alternative chemical processing of organic raw material. Coal, the world's supplies of which is significantly higher than petroleum and gas, is observed in prospect as one of the main raw materials for production of motor fuels and products of organic synthesis. The application of new efficient catalysts and new catalytic processes will allow to overcome many disadvantages of traditional methods of chemical coal processing. It is believed that catalysis would add to coal fuel chemistry the same dramatic changes that were implemented in oil processing in 40-years of this century exactly due to use of appropriate catalysts.
References
- А.И. Камнева Химия горючих ископаемых., Москва, Издательство «Химия», 1974.
- Кузнецов Б.Н. «Катализ химических превращений угля и биомассы», Москва, 1990.
- Г. Н. Макарова, Г. Д. Харлампович Химическая технология твердых горючих ископаемых, М.: Химия,1986.
- В.И. Саранчук, Л.Ф. Бутузова, В.Н. Минкова Термохимическая деструкция бурых углей., Киев, Наукова думка, 1993.
- Л.Лазеров, Г.Ангелова Структура и реакции углей, София, 1990.
- Фальбе Ю.М. Химические вещества из угля. М.: Химия, 1984.
- Левинтер М.Е., Ахметов С.А. Глубокая переработка нефти, 1992.
- Калинко М.К. Тайны образования нефти и горючих газов. М.: Недра, 1981.
- Нефть и нефтепродукты. Масла. Смазки. Присадки, Сборник М., Изд-во Стандарт, 1977.
- Соколов Р.С. Химическая технология В 2 т. Т.2. Металлургические процессы. Переработка химического топлива. Производство химических веществ и полимерных материалов: Учебное пособие. - М.: Владос, 2000. – 447с


