






Abstract
of Qalifing Master's Work
In the course of its activity of the person interacts with surrounding ambience. For its life he extracts from it raw material and brings back waste into it. These pollutants have a different condition. The Surges gas have most importance, since from influence is felt on significant distances from the source of the surge, and pollute not only surrounding ground, but also seas and oceans ruinous act on vegetable organisms. The Amounts surge in the world reach importances 400 mln. tons/year. The Whole in the world in atmosphere is thrown 100-150 mln. tons sulfur dioxide by technology origins. The Surges SO2 beside 1,02 mln. tons form in Ukraine, including 0,36 mln. tons accounts for Donetsk area. The Most amount surge SO2 accounts for turn black and colour metallurgy, heat energy, coal industry (the combustion terrikones)[1]. Dioxid of sulfur (SO2) - is powerfully toxic gas, its maximum single concentration - 0,5 mg/m3. Besides SO2 oxidizing in atmosphere in SO3 , which unites with water ferry and forms the sulfuric acid, which in turn dissolving in water vapour falls out to the land in the manner of acid rains. The Influence of the acid rains on vegetation ruinous. Even weak solutions by chamois of the acid break the processes of the photosynthesis in sheet of the plants, but pine-needles of the conifers perishes at concentrations dioxid of sulfur midair 0,05 mg/m3 [2]. In this connection necessary to warn the arrival in atmosphere dioxid of sulfur.
Purpose of the work: study process of the absorption dioxide of sulfur by compoundes of alki-earth metalls
In Russia in ООО НПО «ДИОМАР» on blazed-chemical combine in Kingisepp (the Leningrad area) produces the sorbents. In composition sorbent enter oxides and hidroxides of ferric, manganese, aluminum: contentses Fe2O3 varies within 15-30%, MnO - 15-25%, Al2O3 - 7-10%. For estimation sorbtion and catalytic characteristic of sorbents is organized row of the studies in laboratory and experienced-industrial condition with attraction specialist. The Called on studies have shown that sorbents show high sorbtion and catalytic activity in all fluid and gas ambience practically. When undertaking the laboratory studies was installed that sorbents clean the leaving gases and from sulfurous anhydride. The Laboratory experiments conducted power of the laboratory ООО НПО «ДИОМАР». Through row, filled by sorbent, missed 100% SO2 and gas-air mixture with contents SO2 10%. The Process of the saturation by SО2 3159 g sorbent (in recalculation on dry material) at drive 168 l SО2 through has occurred in 2 hours 48 minutes. When diluting in 10 once SО2 air and drive gas-air mixture through row at the speed of 1 l/min full saturation gas occurs during 16 h after passage through row 960 l gas-air mixture. When undertaking the chemical analysis in perfected sorbent was discovered presence of the elementary sulfur. This has allowed expecting that Fe-Mn-sorbents possess the catalytic characteristic. [3] In Orel state technical university, Russia, on department of the chemistry were conducted works on estimation thermodynamic balance in systems CaCO3 - CO2 - SO2 and MgCO3 - CO2 - SO2. In work is executed calculation and analysis equal composition of the above named systems under different temperature by decisions of the system of the nonlinear equations of the law acting masses for degrees of the fullness linear-independent reaction. The Installed possibility practically full cleaning gases from compounds of the sulfur when use the limestone or lime in interval of the temperature from 500 to 1300 - 1600 K [4,5].
For reception quantitative estimation, the possibility of the realization to sorption dioxide of sulfur by different compounds, were realized thermodynamic calculations of reactions. Draw a conclusion about possibility of reaction between chemical compoundes, possible using given about change the energy Gibbs in the course of this reactions. The Thermodynamic potential is defined by difference between (ΔH) and (TΔS) factors, acting to system in the course of chemical interaction:
ΔG = ΔH - T * ΔS
The Nature of the change of Gibbs energy allows to judge about principle possibility of the realization of the process. Under ΔG <0 processes can run, under ΔG> 0 processes to run can not. But if ΔG = 0, that system is found able chemical balance. The Reaction possible if she is accompanied the reduction an isobar potential; under room temperature, when importance T small, importance TΔS also small, and change of Enthalpy more TΔS. Than above temperature, that more TΔS, and even endotermical reaction become realized. [6]
The processes of interaction of sulfur dioxide with alkaline earth metal compounds conducted at the facility provided in Figure 1.
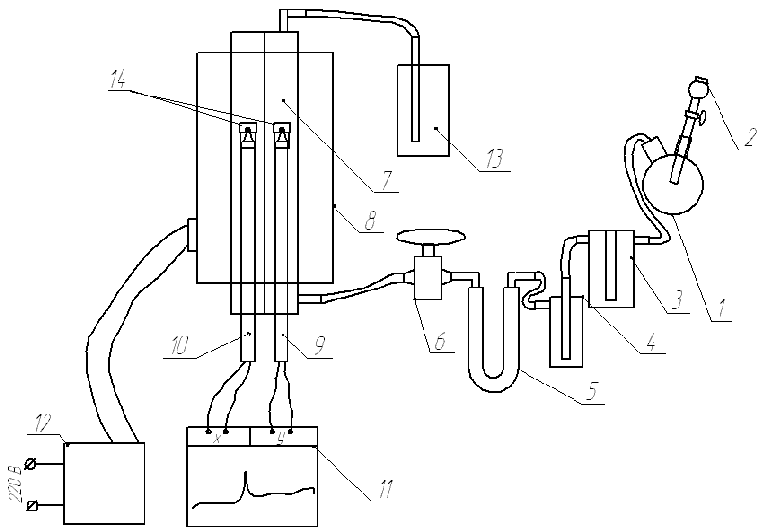
Figure 1 - Labour equipment
Sulfur dioxide are in the bulb 1 in the interaction calculated the number of suspensions sulfita sodium with concentrated sulfuric acid, which is added in small portions through a dropping funnel 2 [7]. Adding just a large number of acids can not, due to the fact that it once formed a large amount of gas, which is due to the high hydraulic resistance system that breaks through the funnel with the acid, and can throw it out. Amount of sulfite calculated on the basis of 10% surplus in relation to the stoichiometric quantity sulfite necessary to obtain the volume of gas capable of 10 times to wash the whole experimental equipment from the air. Hereinafter received gas passes dewatering by concentrated chamois by acid in vial Tischenko 3. For prevention of the arrival of the aerosol of the acid in reactor gas passes trap 4, and then passes the dewatering by silica gel in the tube 5 whereupon enters in reactionary container 7 [8].
The Reactor presents itself two quartz tubes, passing parallel longitudal axis tubular stove. The Maximum temperature of the heating stove 1000oC. Temperature of its heating is adjusted by means of transformer 12 One of the quartz tube is a reaction vessel and the second is required for placement in a «standard» thermocouple. In the reaction vessel is placed under investigation a sample that is placed in the reaction crucible which is placed on the ball «working» thermocouple. Thermocouples are connected differentially to self 11 for fixation of thermal effects of interaction of sulfur dioxide, and the study sample temperature change.
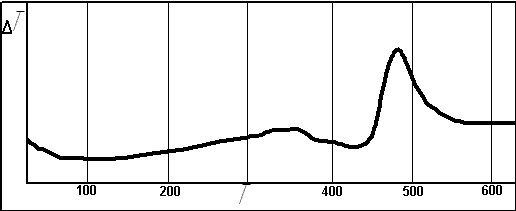
Figure 2 - DTA curve of interaction
The study of absorption of sulfur dioxide by peroxide compounds in the laboratory showed that the interaction starts with little heated and comes with a significant exothermic effect. On differential thermal curve, you can judge the intensity of the process. The growth of mass after the process of absorption was 8% of the original mass of sample. The low level of adsorption can be explained by the interaction of the sample surface and the sulfur oxide to form an inert product, underlying layers of insulating material from the gas phase [10]. Analysis of the IR - spectrum of the reaction products leads to the conclusion that in this sample are anions SO4 2-, SO3 2-. What proves good sorption properties peroxide compounds to sulfur dioxide. The presence of SO4 2- - confirms the lack of completeness of the process. The study of adsorption of sulfur dioxide, calcium peroxide in the laboratory showed that the interaction starts at a temperature of about 430oC and goes with a significant exothermic effect. Figure 2 provides DTA curve of absorption. According to DTA curve of absorption of sulfur dioxide, calcium peroxide can be seen on the intensity of the process. The growth of mass after this process of absorption was 12% of the original mass of sample. Comparing the values of wavelengths of infrared spectrum of the reaction products of tables of the characteristic frequencies can be concluded that in this sample are also anions SO4 2-, SO3 2-. What proves good sorption properties of peroksides compounds to sulfur dioxide.
According to the mass change of samples in the reactions and the spectrogram, it can be concluded that the process of absorption of sulfur dioxide takes place with the formation of peroxides sulphate. What is due to the action of peroxide oxidation-ion with respect to sulfur dioxide.
In conclusion, we can say that the best results for the absorption of sulfur dioxide give substance showing oxidation - remedial properties, and then followed by the United alkaline land showing the basic properties of metals, and finally at the last place on the absorptive capacity of alkaline-earth metals are compounds that exchange is included in their of acid anhydride weaker than the sulfur, the sulfur dioxide molecule.
© Zhebel Anton, Donetsk National Technical University, 2009