
Krivobokov Andrey
Physics-metallurgical faculty
Speciality: Metallurgy of black metals
Theme of master's work:
Method preparation and research reaction kinetics of manganese oxidation in a metal
Scientific adviser: professor Dymnich Anatoliy
The importance of investigation the equilibrium in the metallurgical processes
follows from the fact that the equilibrium state of the reaction confine the any process limit and it is able to manage
the completion of the desired reaction by controlling the factors affecting the equilibrium.
An example of balance, well known in steel production, may be the reaction of manganese oxidation:
[Mn]+[O]→(MnO) (1)
Chemical reaction rate proceeding in one stage, is:
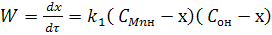 (2) (2)
where k1 - rate constant of direct and inverse reaction;
СMnн и COн - initial or initial reactions of concentration Mn and O in a metal;
х - a parameter which characterizes plenitude of
flowing of reaction.
He is equal to the zero in initial moment of time and increased as far as the accumulation of products of reaction.
After separation of variables x and τ, and integrating with the initial conditions at τ = 0 x = 0, we obtain:
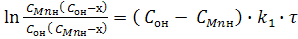 (3) (3)
Solving equation (9) on x, we get:
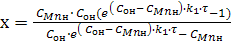 (4) (4)
If in equation (4) make the replacement of x according to equation (6) to
СMnн - СMn and on COн - CO, then after simple transformations we obtain:
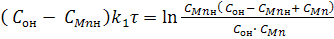 (5) (5)
Introduce new variables:
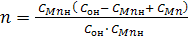
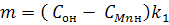
Then equation (5) write in the form:
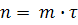 (6) (6)
equation indicates that the parameter n are linearly dependent on time τ. In
coordinates, n, τ the tangent angle of inclination of a straight line m • τ abscissa axis (τ) will be equal to m in
equation (6).
From the expression 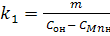 defining the rate constant of direct reaction of manganese oxidation (1).
defining the rate constant of direct reaction of manganese oxidation (1).
Apparent activation energy of reaction (1) can be determin from the Arrhenius equation:
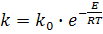 (7) (7)
where k and ko - rate constant of chemical reaction
respectively at temperatures T and T→∞
Е – apparent activation energy of chemical reaction.
I.e. ko - parameter, irrespective of the temperature and is constant for a concrete chemical reaction.
To determine the activation energy (E) necessary to define a constant rate of
chemical reaction by the two temperatures Т1 и Т2:
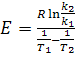 (8) (8)
Define the magnitude of the apparent activation energy (E) parameter ko can
be determine from the expression:
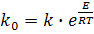 (9) (9)
Thus, parameter ko can define by various temperatures. Under this parameter, it is
can judge by procedural error. Parameter ko is to be the same size by any temperature. According to standard deviation of
this quantity can be defined experiment error.
Literatyre
- Астарита Дж. Массопередача с химической реакцией. Изд-во «Химия», 1971, стр. 224.
- Е.Т. Туркдоган Физическая химия высокотемпературных процессов Пер. с англ. М,: Металлургия, 1985, с.299-305.
- Дымнич А.Х., Троянский А.А. Вопросы тепломассообмена в сталеплавильных процессах - Киев; Донецк: Головное изд-во, 2009, с.25-27.
- Фізико-хімічні процеси позаагрегатного рафінування металу: Навчальний посібник / Зборщик О.М. – Донецьк: ДонНТУ, 2001, с.115-118.
- Конвертерный процесс с долнным дутьем. Арсентьев П.П., Квитко М.П., М.: Металлургия, 1983, 128с, с.35-39.
- Аналитическая химия. Проблемы и подходы: В 2 т: Пер. с англ. / Под ред. Р. Кельнера, Ж.-М. Мерме, М. Отто, Г.М. Видмер. - «Мир»: ООО «Издательство АСТ», 2004, с.412-418.
| 
