Olena Zhyriakova
Sinter Quality
Автореферат > Библиотека
Heinemann Educational books. Agglomeration of iron ores. American Elsevier Publishing Company, Inc., 1973
Sinter Quality. Chemical and physical changes during sintering
D.F.Ball, J.Dartnell, J.Davison, A.Grieve and R.Wild
Sinter Quality
The characteristics of sinter are of paramount importance in that to a high degree they determine the performance of the blast furnace. The objects of sintering are to increase the particle size, to form a strong reducible agglomerate, to remove volatiles and sulphur, and to incorporate flux into the blast-furnace burden.
The first sinter plants were designed and operated with virtually the sole object of increasing the particle size, and the blast furnace was then burdened with an approamount of flux. With the realization that the use of sinter vastly improved furnace performance, the physical properties and chemical constitution came to be examined much more closely.
The introduction of the flux into the sinter mix, rather than separately into the blast-furnace, has very definite advantages in terms of blast-furnace performance. The production of fluxed sinter can be achieved in two ways. For lean ore practice it is possible to blend lime-rich and silicious ores in such proportions as to produce a desired lime/silica weight ratio. Alternatively lime, limestone or dolomite can be added to either high-grade or low-grade silicious ores. This can give rise to the following three practices:
- the production of acid (silicious) sinter where all the flux is added in the blast furnace;
- the production of sinter where sufficient flux has been incorporated for it to have the same basicity as the blast furnace slag and the limestone etc, added in the blast furnace is necessary only to flux the lump ore, pellets and coke;
- the production of sinter where sufficient flux has been added so that there is no need to make further flux additions to the blast furnace.
The production of fluxed sinter is most economic either when all the ore can be obtained as natural fines (i.e. when there are no crushing costs), or when low-grade highly volatile ores are being used so that the advantages gained by sintering more than outweigh the ore crushing costs. With high-grade lump ore it is more economical to crush the ores to a suitable maximum size, screen out the fines and sinter these with sufficient flux to render the whole burden self-fluxing. An alternative method of burdening the furnace would be to use a fairly high proportion of unfluxed pellets together with fluxed sinter. For such practices (i.e. lump ore or unfluxed pellets plus fluxed sinter) to be used a high basicity sinter is required, and for this reason the properties of such fluxed sinters are important. The expression basicity here refers to the weight ratio (CaO + MgO)/(SiO2 + A12O3). If the amount of flux in the finished sinter is sufficient to produce the appropriate amount of slag for the amount of iron produced from that sinter, then this is called self-fluxing sinter, i.e. if all the ferruginous burden charged was self-fluxing sinter then the only other burden material required would be coke. If an excess of flux is added to the sinter mix so that lump or unfluxed pellets can be added to the burden without any limestone being necessary, then this is super-fluxed sinter.
The production of acid sinter is fast declining, and most modern practices produce self-fluxing or super-fluxed sinter. Whilst in the later 1950s it would have been realistic to discuss the quality of fluxed sinters as a special case, this is no longer so.
Chemical and physical changes during sintering
To produce the appropriate quality of product, the sintering operation must be carefully controlled. For this reason the physical and chemical changes which occur from the start of ignition to the end of sintering have been studied in some detail. During the sintering process the bed can be divided into a number of zones; these are shown diagrammatically for pan and moving-grate sintering in Figure 1 and 2. The characteristics of these zones have been investigated by a number of authors. The techniques used are to cool the sintering system and examine sections at various depths. A forced draught of either nitrogen or carbon dioxide can be used for cooling, or the bed can be allowed to cool naturally. A limitation of this technique is that changes can occur during the cooling period and this is likely to be particularly troublesome in relation to the drying zone. The sinter bed after cooling is impregnated with resin and sectioned. Sections of partially sintered zones reveal a number of layers in which various physical and chemical changes can occur.
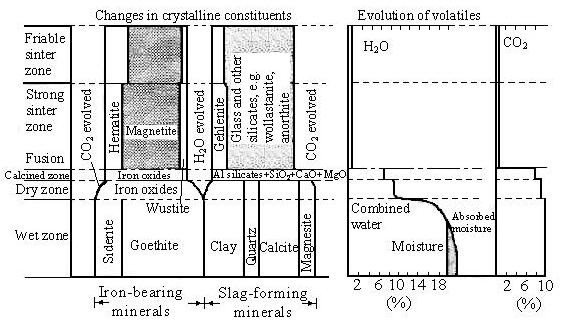
Figure 1 - Changes in constitution during sintering. (Minerals in relative proportions as determined from petrological, X-ray, and chemical analysis. Some clay and goethite may be combined and chamosite. Magnetite is represented separated although it is present in solid solution in siderite and partly as dolomite.)
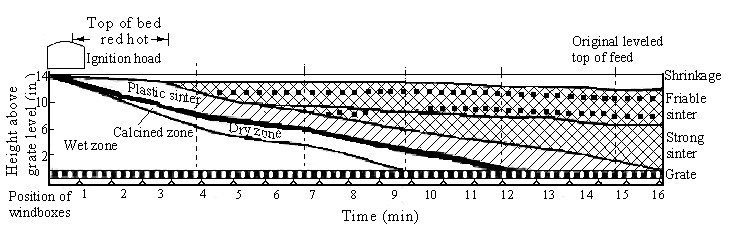
Figure 2 - Zone structure developed on Dwight-Lloyd Strand
A narrow band, not too easily seen in Plate 3, is the region of transition between the coherent sinter cake and the underlying granular portion of the bed; this is essentially the zone of combustion and sinter formation. The first distinct region of unsintered material is the blue-black zone of calcination and reduction. Directly beneath this is the deep red zone of dehydration, and in sharp contrast to this is the light yellow, dry zone. Finally the dark brown, wet zone forms the bottom layer of the bed. With 3 per cent and 6 per cent carbon in the mix, dry granular materials were converted into a coherent mass of sinter within distances of 1.5 in. (38 mm) and 2.75 in. (70 mm) respectively.
The chemical composition changed gradually across the various zones. The ferrous iron increased in the zone of calcination and reduction, and attained a maximum in the freshly formed sinter. Nearer the top of the sinter cake the percentage of ferrous iron decreased as a result of air oxidation. The extent of the increase in ferrous iron and the final amount in the sinter depends upon the amount of fuel used. A substantial amount of sulphur disappeared from the zone of dehydration and, in accordance with general practice, sulphur was eliminated more completely in the low fuel charge.


