Abstract on the Master`s work
Contents
4. Review of literature and elaborations on a theme
Introduction
The combustion of fossil fuels brings in the atmosphere for more than 90% of anthropogenic sulfur dioxide, including about 60% that fall on thermal power plants. It is connected with sufficiently high levels of using sulfur–containing fuel (first of all – high–sulfur masuts and coal) [1].
1 Aim and tasks ⇑
The aim is to study the possibility of using activated chalk, as the sorbent element in the form of briquettes.
To achieve this goal it is necessary to solve the following problems:
- to establish the optimal temperature range of interaction in the system CaCO3 – activator – SO2;
- to determine the quantitative composition of the sorption products that would show absorption properties best of all with respect to sulfur dioxide;
- to compare the studied and measured system’s parameters in the presence of the activator and without;
- to analyze sorption properties of the system in the form of briquettes and in the form of ground powders.
2 Actuality ⇑
Sulfur oxides and formed by the combination of the atmospheric water vapor acids (Н2SO3 and H2SO4) have harmful effects on human health [2], cause the death of coniferous forests, fruit trees, reducing crop yield, acidification of basins. In addition, sulfur oxides are the cause of corrosion of steel structures and destruction of various building materials [2].
At thermal power stations in the Donetsk region at present pre-treatment of flue gases from sulfur dioxide and other combustion products is not provided. Reducing emissions of sulfur oxides is the result of to replacing on power plants sulfurcontaining fuels (mainly masut) with natural gas. However, it can not completely solve the problem of reducing sulfur oxides, given the situation with gas production and its spending on other industries.
Thus, there is the problem of preventing emissions of sulfur dioxide.
3 Science novelty ⇑
It is investigating the possibility of using chalk as the absorber not in the form of powder, but briquettes for the first time. This approach is justified low efficiency of powdered samples, through entrainment of particles of calcium carbonate by flue gases.
Also according to the experimental data, the reaction CaCO3+SO2 →CaCO3+CO2 occurs at low speed, that is the direct using of calcium carbonate will be ineffective. Therefore, we have developed highly effective activators of this process to improve the sorption properties of chalk.
4 Review of literature and elaborations on a theme ⇑
All known ways of organizing the process removing sulfur dioxide from flue gases at present can be classified as follows:
- absorptions [1-7], in which sulfur dioxide chemically bounds into the drilling fluid through molecular attraction [2,4,5];
- adsorptions [1-7], in which the binding of sulfur dioxide with a surface of solid material by purely physical forces of interaction;
- chemisorptions at which the chemical bonding with a solid material.
The above methods can be divided into wet and dry depending on the phase in which the process of binding of sulfur dioxide [1-7].
There were created inventions for cleaning from sulfur dioxide, which mainly refers to the field of wet gas cleaning in Russia over the past 10 years.
Institute of Sciences of Ukraine developed ammonia cleaning method [4, 8].
This problem was considered at the Department of Applied Ecology and Chemical Technology. Here activators have been developed based on salts of metals, which greatly increase the efficiency of absorption of sulfur dioxide with oxides of calcium. Also there was search for activators of calcium carbonate in the department.
5 Received results ⇑
There was recorded curve by differential thermal analysis (DTA) in order to study the interaction of SO2 in the system CaCO3 – activator. Curves of differential thermal analysis are shown in Figure 1.
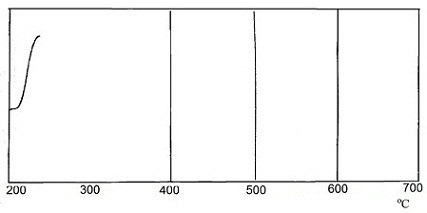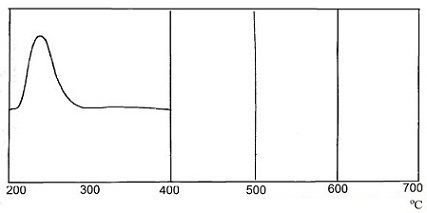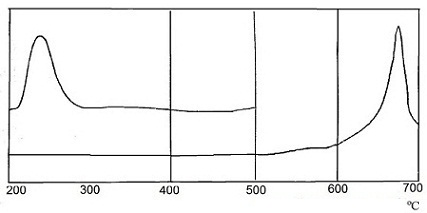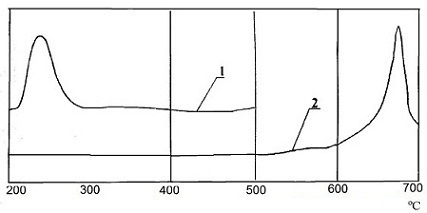
The heating was carried out at 12 оС/min in a glass cup. α-oxide of aluminium was used as reference material. Recording the thermograms was carried out with sensitivity to the axis X – 1 mV/cm, on axis Y – 0,25 mV/cm.
The results of the absorption of sulfur dioxide with calcium carbonate are shown in Table 1.
| System | Hanging carbonate | Activator content | Increment of mass SO2 | Capacity for SO2 mole / mole | |||
| g | mole | g | mole | g | mole | ||
| Activated | 0,2030 | 0,0020 | 0,0234 | 0,0003 | 0,0960 | 0,0015 | 0,8866 |
| Nonactivated | 0,2440 | 0,0024 | 0 | 0 | 0,1366 | 0,1366 | 0,8748 |
Conclusion ⇑
From the experimental data shows that the completeness of the interaction of sulfur dioxide with calcium carbonate is about 90%. However, in the presence of the activator interaction begins at a lower temperature – 215 оС, than interacting with non–activated calcium carbonate, where the absorption process occurs at a temperature (600÷700) оС. This fact is very interesting for industrial enterprises.
Also there was been established activation mechanism and a mathematical model of the process.
In writing this abstract master's work is not completed yet. Final completion: December 2011. Full text of the work and materials on the subject can be received from the author or his manager after that date.
Literature ⇑
- Пугач Л.И. Энергетика и экология: Учебник. – Новосибирск: Изд-во НГТУ, 2003. – 504 с.
- Алферова, Т. В. Экология энергетики: курс лекций / Т. В. Алферова,О. М. Попова. – Гомель, 2008. – 123 с.
- Маляренко, В.А. Введение в инженерную экологию энергетики: Учебное пособие. – Харьков: САГА, 2008. – 185 с.
- Носков А.С., Пай З.П. Технологические методы защиты атмосферы от вредных выбросов на предприятиях энергетики.– Новосибирск: АН СССР, 1996. – 155 с.
- Носков А.С.,Савинкина М.А., Анищенко П.Я. Воздействие ТЭС на окружающкю среду и способы снижения наносимого ущерба. – Новосибирск: АН СССР, 1990 – 590 с.
- Родионов А.И. Технологические проблемы экологической безопасности/ А.И. Родионов, В.Н. Клушин, В.Г. Систер. – Калуга: изд-во Н.Бочкаревой, 2000. – 800 с.
- З.М. Боброва, О.Ю. Ильина, Т.Ю. Тюрина Изучение методов улавливания диоксида серы в отходящих газах. [Электронный ресурс]. – Режим доступа:
http://ecology.ostu.ru/index.php?option=com_content&task=view&id=91&Itemid=51 - Гладкий А.В. Современное состояние и перспективы мирового развития методов десульфуризации отходящих промышленных газов // Промышленная и санитарная очистка газов. – М.: ЦИНТИХИМНЕФТЕМАШ. 1990. – 28 с.
- Кропп Л..И., Новоселов С.С. Пути сокращения выбросов двуокиси серы с дымовыми газами ТЭС. – М.: 1986. – 56 с.
- Способ очистки дымовых газов от диоксида серы – Патент РФ №2016634. [Электронный ресурс]. – Режим доступа: http://ru-patent.info/20/15-19/2016634.html
- Способ очистки отходящих газов от диоксида серы – Патент РФ №2074014. [Электронный ресурс]. – Режим доступа: http://ru-patent.info/20/70-74/2074014.html
- Cпособ очистки газов от фтористого водорода и диоксида серы – Патент РФ №2074015. [Электронный ресурс]. – Режим доступа:
http://ru-patent.info/20/70-74/2074015.html - Способ очистки отходящих дымовых газов от оксидов серы и азота – Патент РФ №2085262. [Электронный ресурс]. – Режим доступа:
http://ru-patent.info/20/85-89/2085262.html - Виселесов Н.Г., Большунов В.Г. Утилизация промышленных сернистых газов. – Киев: Наукова думка, 1990. – 136 с.
