Abstract
Maintenance
- 1.Relevance, purpose and objectives of the master’s work.
- 2. The solid residue of low-temperature pyrolysis of tires and technical analysis
- 3. General description of adsorption processes
- 4. The results obtained
- Conclusion
- References
1.Relevance, purpose and objectives of the master’s work.
Sorption is one of the universal methods of cleaning and purification of waste water from the dissolved organic compounds of natural and unnatural sourse [1].
As sorbents used various artificial and natural porous materials, but more common is the activated carbon, which is limited to the use of high cost. In recent years the tendency to search for new carbonaceous materials for production of sorbents.
Known methods for producing sorbents of lignin (wood processing solid waste), waste oil, used automobile tires, agricultural and other wastes. For example, such a method as a low-temperature pyrolysis, can be obtained from the tires up to 45 % solid residue containing about 90 %. carbon, which in its properties is a good sorbent material. On the other hand, this method allows you to recover a significant amount of waste tires that are difficult to neutralize other environmentally friendly methods [2].
The purpose of master's work is an experimental study of the sorption properties of qualitative and quantitative characteristics of the solid product of pyrolysis of used tires.
Tasks:
1. Carrying out technical analysis of solid residue.
2. Experimental determination of sorption capacity of the solid residue with respect to iodine and organic dyes: methylene blue, methyl orange under static conditions by standard methods.
3. Research of sorption properties with respect to some organic pollutants and heavy metal ions.
2 Low-temperature pyrolysis solid residue tires and technical analysis
The problem of waste, including rubber tires worn out, in today's society remains important, despite the development of production technology of new environmentally friendly products. Storage and recycling and waste disposal cost effective and environmentally safe, since the long-term storage, they can release into the environment of substances that can disrupt the ecological balance [3]. One of the promising methods of recycling is a low-temperature pyrolysis – thermal decomposition of organic material without air at a temperature (400 ? 500) °C. At present, passed the test pilot plant forprocessing low-temperature pyrolysis of used tires [4]. In addition to the pyrolysis of liquid and gaseous products, a solid residue, which can be used as a sorbent.
The results of the technical analysis suggests that the key indicator - the carbon content of 83,8 %, therefore, the solid residue can be used as a sorbent.
3 General description of adsorption processes
There is currently no general theory, which is quite correct to describe all kinds of adsorption on different adsorbents and different surfaces of the interface. Therefore, considering the most popular theory, which (despite the large number of assumptions) allow a qualitative level, to get an idea of such a complex process as adsorption. This theory of molecular adsorption of the Langmuir theory of multilayer adsorption and the generalized theory of Brunauela, Emmett and Teller. The interaction of molecular components of the solution with the atoms or molecules of a solid surface leads to the fact that the molecules of these components are kept for some time at the interface. The higher the energy of interaction of molecules with the surface of the adsorbent material, the longer the molecule is at the interface, ie exists in the adsorbed state.
In the adsorption of smaller molecules, usually even at high equilibrium concentrations, specific adsorption does not reach the limit, but gradually, though slowly, continues to change. The conditions of adsorption equilibrium is well expressed by the empirical Freundlich equation (the equation of a parabola) (figure 4.1) [5]:
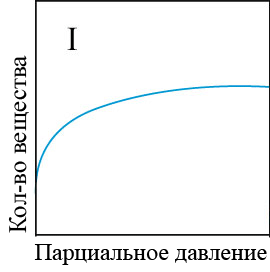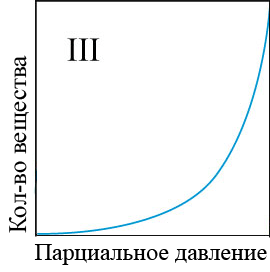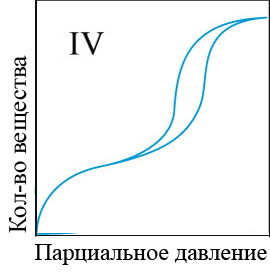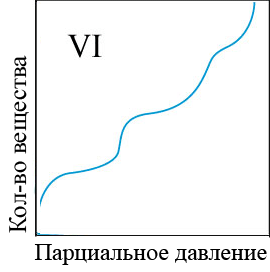
Figure 4 – Types of adsorption isothermsи
Г = αС1/k, (3.2)
where α and 1/k - constants that define the curvature of the parabola.
When the equilibrium concentration equal to unity, the value of specific adsorption of F is equal to a constant ?. The larger ?, the greater the specific adsorption of the substance. Consequently, the value of ? may be conditional measure of activity of the adsorbent at low concentrations of substances absorbed from the solution. To determine the adsorption constants ?, k the adsorption curves constructed in the evident and logarithmic coordinates and perform the appropriate calculations[6].
4 Results
To confirm the possibility of using the solid residue as a sorbent were conducted its tests by standard methods [7]. According to [7] determined the adsorption activity with respect to methylene blue, methyl orange and methyl red. Research carried out under static conditions - the sorbent was placed in a closed system. When processing the results of the magnitude of the adsorption capacity of the solid residue of pyrolysis of tires was 60-80 % of the adsorption capacity of activated carbon, given the standard [7].
For this batch of coal weighing 0.1000 g was placed in a conical flask of 200 ml, 25 ml of added dye, covered with a lid and shaken for 20 min. After this, the optical density at fotoelektrokolorimetre with blue filter and a wavelength of 400 nm in cuvettes with a distance between the working faces of 10 mm. As a reference solution using distilled water. According to the obtained optical densities based on the calibration curve determined by the residual concentration of the dye.
Adsorptive activity was calculated by the formula:

where C1 - concentration of the dye stock solution, mg/dm3;
C2 - the concentration of the dye solution after contact with activated carbon, mg/dm3;
K - coefficient of dilution;
m - mass of activated carbon sample, g;
0,025 - the amount of the dye solution, participating in the study, dm3.
To confirm the possibility of using the solid residue as a sorbent for determining the type of adsorption isotherms were investigated the sorption properties of the solid residue and defined constants in the equation of Freundlich. To do this, a series of experiments in which the varied initial concentrations of methylene blue and methyl orange MB MO in the ranges given in Table 4.1.

As a result of the calculations based on data in Fig. 4.1-4.2 adsorption constants were determined and the resulting sleduyuschin equation:
a) methylene blue - Г = 1,000 С0,466;
b) for methyl orange - Г=0,282 С0,424;
The resulting coefficients in the equations indicate sufficient sorption properties of the solid residue [8].
An important characteristic of carbonaceous sorbents is the sorption capacity for iodine, which according to [9] shall be for a grade coals OC – 30 % for BAU – 60 %.
Adsorption activity by iodine balance study was 73 %, which corresponds to the adsorption activity of well-known brands of coal.
Findings
In the treatment technologies along with the known sorbents such as activated carbon, silica gel can be used sorbents derived from waste products. One such waste is the solid residue of pyrolysis of used tires, which by its characteristics and properties are not inferior to well-known brands of coal.
In the pilot study, data were obtained on the basis of which we can talk about the feasibility of using the product of pyrolysis for waste treatment of organic pollutants.
In the future we plan to study the sorption properties of solids under dynamic conditions, namely the removal of wastewater organic pollutants and heavy metal ions. Will be conducted investigating the possibility of sewage treatment plant on the model coke plant.
References
- Электронная статья. Активированный уголь, очистка воды и воздуха [Электронный ресурс]: http://www.activcarbon.com.ua
- Шендрик, Т.Г. Утилизация нефтяных отходов с получением активированных углей / Т.Г. Шендрик, Л.В. Пащенко, В.А. Кучеренко. В.В. Симонов // Углехимический журнал. – 2005. № 5. – с. 27-31.
- Миллер Р. Теория переключательных схем / Р. Миллер. – М.: Наука, 1971. – Том 2: Последовательностные схемы и машины. – 304 с.
- Электронная статья. Утилизация шин и экология [Электронный ресурс]: http://www.utilrti.ru
- Булавин А.В., Пашкевич В.Н. Переработка отработанных автомобильных шин методом низкотемпераьурного пиролиза. / Научные труды Донецкого национального технического университета. // Химия и химическая технология. Выпуск 95. – Д:Лебедь, 2005. – с. 98 – 102.
- Когановский А.М. Адсорбция и ионный обмен в процессах водоподготовки и очистки сточных вод. / А.М. Когановский. – К.: Наукова думка, 1983. – 240 с.
- Смирнов А.Д. Сорбционная очистка воды./ А.Д. Смирнов. – Л.: Химия, 1982. – 168 с.
- Адсорбционная технология очистки сточных вод / А.М. Когановский, Т.М. Левченко, И.Г. Рода. – К.: Техніка, 1981. – 176 с.
- ГОСТ 4453 – 74. Уголь активный осветляющий древесный порошкообразный. М.: Государственный комитет стандартов, 1974. –11 с.
- Цыбульская К.В., Трошина Е.А. Изучение возможности использования твёрдого продукта пиролиза автомобильных шин. / Сборник докладов Международной научно-практической конференции молодых ученых и студентов «Современные экологически безопасные и энергосберегающих технологи в природопользовании» . – ч. 2. – К: КНУБА, 2011. – с. 53 – 55.
- ГОСТ 6217 – 74. Уголь активный древесный дробленый. – М.: Государственный комитет стандартов, 1974. – 6 с.


