Abstract
Contents
- Introduction
- 1. The relevance, purpose and tasks of the research
- 2. Characteristics of synthetic oxygen carriers
- 2.1 Сonnections of cobalt(II)
- 2.2 Research of the mixed-cobalt system
- 3 Realization of the experiment
- 4 Results
- Conclusion
- References
Introduction
Among the various coordination complex compounds there are particularly interesting transition metal compounds with molecular oxygen, and the problem of a reversible interaction with O2 metal complexes is extremely important. This is basically connecting with due to the fact that on the basis of this type of the coordination compounds catalysts for the oxidation of some organic compounds with high performance can be produce. Along with this, the study of the processes of the coordination compounds oxygenation d-elements is important from the point of view of studying the structure and properties of molecular oxygen carriers in living organism[1].
Ability to coordinate molecular oxygen complexes is possess a certain structure of most elements of the first transition series, as well as d-group VIII elements in lower oxidation. Efficiency of interaction complexes of these metals with molecular oxygen is determined geometric structure complexes composition nearest coordination sphere, nature and donor properties of the ligand occupying the trans position. One of the specific features of the oxygen octahedral complexes is the ability to adjust the properties of the coordinated oxygen molecule by modifying the trans-ligand.
These requirements are to some extent responsible low spin planar complexes cobalt(II) schiff bases, the coordination sphere of which contains two or four nitrogen atoms, and as the ligand acts trans N-heterocyclic base. Coordination compounds with molecular oxygen are essential for modeling biological processes as well as elucidating the mechanisms of redox processes in a homogeneous medium [1].
1. The relevance, purpose and tasks of the research
Relevance of the topic is that the ability of complex compounds of cobalt reversibly attach an oxygen molecule is important from an environmental point of view. Since these compounds are active centers in models of complex biological compounds (haemoglobin, myoglobin, gemeritrin, hemocyanin) and can therefore be regarded as the natural oxygen carriers. Already developed drugs such as perftoran perfukol on the basis of hydrocarbons, but they are not perfect and have a number of drawbacks that hinder their use in clinical settings. To solve this problem need to search for more optimal synthetic oxygen carriers [2].
The purpose of research is to study the complexation reactions in the system cobalt(II)-alanylalanine-dipyridyl as an inert atmosphere and in the presence of oxygen. Determining the conditions of formation of oxygenated complexes play an important role in modern medicine.
For gaining end next tasks are put:
1 – opredelit to define the constants of equilibrium of all stages of process;
2 – postroit to build the crooked distributions;
3 – to expect equilibrium concentrations in the investigated system;
4 – to compare the got results to present in literature with the purpose of choice of the most optimal terms of realization of research.
2. Characteristics of synthetic oxygen carriers
Some coordination compounds of transition metals in low oxidation can reversibly bind oxygen molecule.
Close to natural transporters O2 metal complexes were first transition series of low oxidation state in (Mn(II), Fe (II), Co(II), Ni(II), Cu(I)). They (especially the compound Co(II)) are capable of reversibly oxygenated aqueous solutions and have the composition of the inner coordination sphere, similar to natural active centers [1, 2, 5].
Interest in the coordinated activation of molecular oxygen as part of possible formation of short-lived intermediates in homogeneous catalytic reactions autoxidation. Of particular importance for elucidating the mechanisms of reactions are studies O2 reactivity in oxygenated complex. Study of the reactions of the intermediate complex mechanism allows to present homogeneously-catalytic reaction in the form of separate stages. Consequently, the compounds of transition metals with O2 are crucial for the modeling of biological processes, and also for finding out of mechanism and search of catalysts of the ORP processes.
However, the role of transition metal complexes with O2 is not limited to purely chemical, biological and medical aspects. They may serve as the source of pure oxygen for various technological purposes. Coordination compounds capable of binding O2, can serve as indicators of oxygen. Compounds capable to attach and activate O2, can serve as catalysts for reactions of industrially important products of oxygen electroreduction catalysts, biologically active compounds, and also be used in gas purification from oxygen impurities.
Currently coordination compound with molecular oxygen are known for all 3d-transition metals of low oxidation state [3, 6]. On the course of redox processes between O2 and metal ions(II) mid — 3d-transition series affected by the nature of the ligands of the inner coordination sphere, primarily nitrogen containing. Recovery coordinated oxygen molecule to suroxide or superoxide ion often provides its reversible binding of the metal complex. Indeed, at present the greatest number of coordination compounds obtained by molecular oxygen ions to Co(II), Fe(II) and Mn(II) [3].
Artificial oxygen carriers — are chemical compounds that are used to increase the amount of oxygen in the blood. The artificial oxygen carriers can be used, where:
– real blood is not available,
– there is a risk of infection some infection,
– no time to check the compatibility of the blood donor and recipient.
However, these products are rarely used, they are constantly being improved, require spending more research and clinical trials. Oxygen compounds of metals reversibly up oxygen can serve as models of natural molecular oxygen carriers. The synthetic carriers of molecular oxygen present and undoubtedly technical interest:
– are a potential working material for the production of oxygen from the atmosphere under mild conditions;
– can serve as catalysts in homogeneous catalytic redox reactions (as in the coordination of molecular oxygen is its activation);
– used to increase the efficiency of the positive electrode in a fuel cell;
– for deep cleaning liquids and gases oxygen, serve as antioxidants [2].
Of the currently known complexes reversibly binding molecular oxygen, most are complex compounds of cobalt with various ligands: schiff bases, amines, amino acids, porphyrins, etc.
2.1 Сonnections of cobalt(II)
The special position of metals of the first transition to the ability to form coordination compounds with O2 takes cobalt. Most currently known 3d-coordination compounds of transition metals with O2 are compounds of cobalt ratio Co:O2 = 2:1 and 1:1. This section discusses the main types of coordination compounds of cobalt with O2. Several types of ligands, which in coordination with the Co(II), creates the conditions for the formation of reversible complexes with O2: schiff bases, porphyrins, amines, macrocyclic amines, polyamines, amino acids, chelators, peptides, polypeptides. Consideration of the main types of coordination compounds of cobalt with O2 shows that most of them have a coordination number is six. Ion oxygenated cobalt complexes with the ligand is linked mainly via the nitrogen atoms (usually at least three), and oxygen.
Thus, ions of 3d-transition metals of low oxidation state form a coordination compound capable of reversibly reacting with O2. This property depends on the nature of the ligands, the inner coordination sphere of the metal ions. The composition of the nearest environment of the metal ion includes preferably the nitrogen atoms and oxygen atoms, phosphorus, and sometimes sulfur. Known mononuclear (M:O2 = 1:1) and dual-core (M:O2 = 2:1) coordination compounds of 3d-transition metals with O2. More common in recent metal end of the series. Most synthetic oxygen carriers is the coordination compounds of Co(II). On this basis the most efficient vectors were obtained in the solid state O2 (coordination compound Co(II) schiff bases) and solutions [1].
The mechanism of formation of the oxygen complexes. In general, the education of the oxygen cobalt complexes may be submitted by the following scheme (ion charges are omitted) (figures 2.1):

Figures 2.1 — The mechanism of formation of the oxygen complexes
(animation: 10 frames, 7 reiterations, 9 kilobytes)
Equation (1) corresponds to the formation of mononuclear of the oxygen complex. If you do not create favorable conditions that stabilize mononuclear of the oxygen complex, then it turns into a binuclear complex (equation (2)). By stabilizing factors that prevent the formation of binuclear complexes include: the use of donor solvents with low dielectric constant; spatial factors that inhibit dimerization of the complex, the lowest temperature of the solution, a low concentration of the complex; as useful as ligands of the macromolecule, reduces the possibility that the formation of O2 – bridge between two central ions of cobalt(II). In aqueous solution, the formation of the oxygen complexes almost always leads to the formation of dinuclear complexes [3].
2.2 Research of the mixed-cobalt system
Initiating studies of the formation of mixed-oxygenated complexes of cobalt(II) was put into operation in 1980. 2,2'-dipyridyl was synthesized in the late 19th century Blau. Molecule 2,2'-dipyridyl (Dipy) in figure 2.2:
Figure 2.2 — Molecule 2,2'-dipyridyl (Dipy)
Dipeptides — compounds which consist of two amino acid residues linked together by peptide bond. In the aqueous solution exist in the form of dipeptides, zwitterions, in which the carboxyl group is deprotonated and the protonated amino group (figures 2.3):
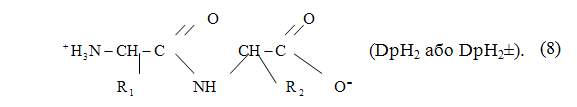
Figure 2.3 — Dipeptide molecule
3 Realization of the experiment
To study the complexation and oxygenation in the system cobalt(II)-alanylalanine-dipyridyl-oxygen was applied pH-method. In the course of the experiment conducted titration mixture of aqueous solutions of salts of cobalt(II), and dipyridyl alanylalanine (series of dipeptide alanyl) at a molar ratio of 1:1:1 and 1:1:2, with the ionic strength of 0,1 (KNO3), t = 25°C, in an inert atmosphere and air atmosphere. Apparatus for carrying out the pH-method studies presented below.

Figure 3.1 — Apparatus for carrying out the pH-method studies
4. Results
During the study was composed of equations for mixed-system Co(II)-Alaala-dipy-O2, with pH-method conducted research monodipiridilovogo complex mixture of cobalt(II) and alkali alanylalanine in air. General scheme of the complexation equilibria and oxygenation system mixed — cobalt(II)-alanylalanine-dipyridyl-oxygen in the air includes 32 reaction. Necessary follow-up study of properties obtained in the system Co(II)-Alaala-dipy-O2 binuclear cobalt to determine optimal conditions for their education for future use as synthetic oxygen carriers.
Conclusion
During the operation was considered the formation of complexes of cobalt and oxygenation system in mixed cobalt(II)-dipyridyl-alanylalanine. Ph values were determined in the system in an inert atmosphere and in an air atmosphere. In the future will be made calculation of the equilibrium constants, the construction of the distribution curves and calculation of equilibrium concentrations in the studied system. Obtained Constants can be used as independent reference values, and data on the highest concentration of the complex at each specific pH can be used to obtain the desired complex — a synthetic oxygen carrier.
References
- Братушко, Ю.И. Координационные соединения 3d-nepexoдных металлов с молекулярным кислородом / Ю.И. Братушко. — К.: Наук. Думка, 1987. — 168 с.
- Ганнова Ю.М., Фурман О.В., Катишева В.В. Визначення умов утворення октанованих комплексів в системі кобальт(II)-гліцилаланін-дипіридил // Наукові праці ДонНТУ. Серія: хімії і хімічна технологія. — 2013. — Вип. 2(21) — с.83–91
- Гринберг, А.А. Введение в химию комплексных соединений / А.А. Гринберг. — М.: Химия, 1971. — 371 с.
- Паладе Д.М. Кинетика и механизм образования оксигенированных комплексов кобальта // Коорд. химия. — 1992. — Т. 18, — №7 — С.729–749.
- Паладе Д.М. Определение кобальта в его комплексных соединениях // Ж. аналит. химии. — 1966. — Т.21. — С.377–378.
- Паладе Д.М., Линькова B.C. Чудаева Г. В. Оксигенация бисдипиридилового комплека кобальта(II) // Ж. Неорган. химии. — 1982. — Т.27, — № 9. — С. 2311–2315.
- Скурлатов Ю.И., Пурмаль А.П. Связывание О2 комплексами Со2+ с 2,2'-дипиридилом. — № 1474 — 70 Деп.: М. — 1969. — 19 с.
- Скурлатов Ю.И., Пурмаль А.П. Связывание О2 комплексами Со2+ с 2,2'-дипиридилом // Ж.физ.химии. — 1970. — Т.44. — С. 1364–1372.
- Эйхгорн, Г. Неорганическая биохимия / Г. Эйхгорн. — М.: Мир, 1978. — 737с.
- Ганнова Ю.Н., Шаповалов В.В., Фурман Е.В. Оксигенация в смешаннолегандной системе кобальт(II)-фенантролин-аланилаланин // Наукові праці ДонНТУ. Серія: хімії і хімічна технологія. — 2011. — Вип. 2(18) — с.15–21
- Краткая химическая энциклопедия. — М.: Советская энциклопедия, 1964 — Т.З. — 903 с.
