Abstract on the theme of graduation work
Introduction
Adsorbents are highly dispersed natural or artificial materials with a large specific surface on which adsorption of substances from gases or liquids in contact with it occurs. Adsorbents are used to purify water from metals and impurities, in gas masks, as catalyst carriers, in medicine to absorb gases and poisons.
Adsorption properties of adsorbents depend on the chemical composition and physical condition of the surface, on the nature of porosity and specific surface area (surface per 1 g of substance). Nonporous adsorbents (ground crystals, fine crystalline precipitates, smoke particles, soot, aerosols) have specific surfaces from 1 m2/g to 500 m2/g. the Specific surface of porous adsorbents (silica gels, activated alumina (alumina), aluminosilicate catalysts, activated carbons) reaches 1000 m2/g. Porous adsorbents are obtained by creating a network of pores in coarse solids by chemical action.
Adsorbents are also used as fillers for polymers, for chromatographic separation of chromatographic mixtures in chromatography, in oil refining (reforming, hydrotreating, hydrocracking), and in petrochemicals for refining of oil products (oil, gasoline, etc.). e.) and gases, adsorption cleaning of oils, primarily transformer oils, from acids – products of oil oxidation, as static dehumidifiers for preservation of devices and equipment, as well as in high-vacuum equipment for sorption pumps.
The most common adsorbents are active (activated) coals, which are produced by thermal destruction of substances containing carbon. Most often, birch is used as a raw material. Birch active coals (BAС) can be used for sorption processes in the food industry and medicine – this indicates the high quality of these adsorbents. But it should be remembered that birch is a long-term source of raw materials, not to mention the deterioration of the ecological condition of the regions where the mass felling of these trees takes place.
1. Theme urgency
Adsorbents can be a variety of materials with a high specific surface area: porous carbon (the most common form is activated carbon), silica gels, zeolites, as well as some other groups of natural minerals and synthetic substances. High-quality adsorbents are obtained by pyrolysis of low-tar tree species, such as birch, which is certainly not acceptable from both social and environmental points of view. Therefore, at present, methods of obtaining active coals from alternative raw materials are being actively developed.
2. Goal and tasks of the research
The main objective of this work is to develop a method of obtaining new adsorbents from rapidly renewable vegetable raw materials, as well as carbon-containing food waste.
Main tasks of the research:
- Consider the possibility of obtaining adsorbents from unconventional raw materials.
- Get samples of adsorbents from vegetable raw materials.
- Study the influence of salts on the process of obtaining activated coals.
- Find out about the possibility of using the obtained samples instead of traditional activated coals.
3. Main part
Adsorption – process of spontaneous redistribution of system components between the surface layer and the volume phase.
Adsorption is a mechanism for fixing substances in molecular or ionic form on the surface of materials with certain properties. This process is usually accompanied by the transfer of a substance in a state of true or colloidal solution to a solid-phase surface. The adsorption properties of such solids are primarily determined by their specific surface, measured in square meters per gram of adsorbent substance.
Adsorption consists in capturing the surface of microporous adsorbent (activated carbon, silica gel, zeolites) molecules of harmful substances. One of the best adsorbents is activated carbon, which in 1 g contains up to 1600 m2 of surfaces.
Aside from the total surface area of the adsorbent, the adsorption level is also determined by the character of the bond between the adsorbent and the adsorbed substance. That is, the free energy of interaction between the adsorption center of the sorbent material and a part of the molecule interacting with it [1].
Primarily the adsorption value is influenced by the Van der Waals forces [2] as well as the electrostatic attraction forces.
It also means affinity between molecules, for example, aromatic hydrocarbons and carbon with graphite structure, which has repulsive forces with organic compounds of other structure. The time of contact between the adsorbing agent and the waste water deposited on it, which determines the degree of reaching the adsorbent surface, is also important. The mass of the adsorbed substance per unit of the sorbent surface is determined by its concentration in the aquatic environment. In the equilibrium state, the molecules adsorbed on the surface are constantly changing in places, which is described in some approximation by Freundlich's law [3].
Distinguish between six (five) basic types of adsorption isotherms (Fig. 1). Type I is typical for microporous solids with a relatively small fraction of the outer surface. Type II indicates polymolecular adsorption on non-porous or macroporous adsorbents. Type III is typical for non-porous sorbents with low interaction energy of adsorbent adsorbate. Types IV and V are similar to types II and III, but for porous adsorbents. Isotherms of type VI are typical for non-porous adsorbents with homogeneous surface [4].
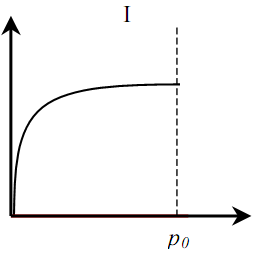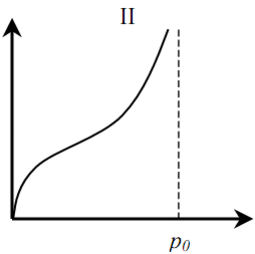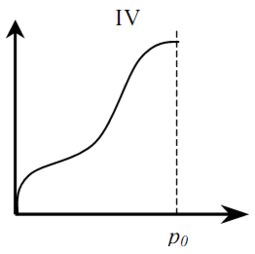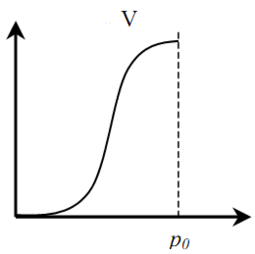
Figure 1 – Types of adsorption isotherms
Scientist Kiselev divided the adsorbents into three classes [5] according to the following features:
Class 1 – there are no ions or positively charged groups on the surface, e.g. graphite soot. Class 2 – there are concentrated positive charges on the surface, for example, groups of OH on hydroxylated oxides. Class 3 – there are concentrated negative charges on the surface, for example, =O, =CO.
In practice, adsorption methods are usually used to determine the specific surface and porous structure of only highly dispersed solids or systems with developed porosity.
Usually the particles of highly dispersed powder – primary particles – stick together in more or less dense secondary particles under the influence of surface forces. If the contacts between adjacent particles are weak, these aggregations, called aggregates, can break down. At elevated temperatures or under pressure, the primary particles are combined into stronger secondary particles, the so-called agglomerates [6].
Adsorption properties depend on the pore size range – each size has its own characteristics. In micropores, the potential for interaction with adsorbed molecules is much greater than in wider pores, such as those of meso- and macropores – this is due to the proximity of the pore walls. The value of adsorption at this particular relative pressure is also higher. Capillary condensation occurs in medium-sized pores – in mesopores. Unfortunately, it is not always possible to study the isotherm of adsorption – macropores are too wide for this purpose [7].
The most important raw material for the production of active coals is wood (in the form of sawdust), charcoal, peat, peat coke, some hard and brown coals, as well as semi-coke brown coals.
Finely shredded wood waste, carbonized in rotary kilns or machines with moving layers. Lumpy and pelletized coals, as well as pressed wood dust and binder products, are activated in mine and rotary kilns with water vapour or carbon dioxide at 800 °C. In the process of activation, the pore volume, the specific surface of the sorbent increases and the ratio between the volumes of micro-, meso- and macropores changes. The rate of gasification of surface carbon in the process of activation depends on the degree of structural ordering of carbon material. Carbon is most easily and quickly gasified in disordered areas of the carbon surface. Prepared with a binder molded coal must be thermally processed before activation at a temperature of about 5000 °C, the binder in these cases is partially carbonized. The quality of active carbon depends on the properties of the initial carbon-containing material and on the activation mode. Characteristics of the degree of activation of coal is soot, i.e. the percentage of burned coal in relation to its initial amount. Active charcoal is characterized by a high degree of purity and thin porosity [8].
The number and size of pores produced are determined by the nature of the raw materials and the regime parameters of the heat treatment process. The speed of raw material heating is of great importance. The total volume of pores, as well as the number of large pores (macropores) increase significantly with the increase in the rate of heating of raw materials. Slow heating rates are realized in pyrolysis technologies in reactors with a fixed layer of raw materials. More efficient pyrolysis technologies are based on the use of shredded raw materials and reactors with so-called fluidized or fluidized bed: small particles of raw materials, carried away by the gas flow, seem to be in a boiling state. The advantage of fluidized bed reactors is the high rate of mass and heat transfer, which provides increased intensity of pyrolysis process in comparison with the technology of pyrolysis in a fixed layer of raw materials. The pore volume and the radial distribution of pores can also be adjusted by changing the duration of the pyrolysis process. In reactors with fluidized bed, the duration of stay of the crushed raw material particles in the pyrolysis zone varies from a tenth of a second to several minutes [9].
Conclusion
Magister's work is devoted to the actual task of searching for new adsorbents on the basis of rapidly renewable vegetable raw materials. Within the limits of the spent researches it is executed:
- In the course of writing the master's work, the data on the methods of obtaining adsorbents from plant raw materials, in particular starch, were studied and systematized.
- The methods of spectrophotometric analysis are studied.
- It has been proven that starch can be used as an easily renewable source of raw materials for the production of activated carbon.
- It is established that starch exhibits its adsorption properties when interacting with microsilica.
- It has been determined that the addition of starch with an activator of salts such as AlCl3 and NaCl reduces the content of water-soluble substances in pyrolysis products.
- It has been studied that the obtained adsorbents can be used for sorption of phenol from aqueous solutions.
Production and application of carbon adsorbents on the basis of natural mineral and organic components is expedient, as they can replace the existing adsorbents produced on the basis of wood, which will significantly improve the state of the environment in the region.
References
- Грег, С. Адсорбция, удельная поверхность, пористость/ С. Грег. – М.: Мир, 1984. – 306 с.
- Фенелонов, В. Б. Пористый углерод / В. Б. Фенелонов. – Новосибирск.: Химия, 1995. – 513 с.
- Кинхле, Х. Активные угли и их промышленное применение / Х. Кинхле, Э. Бадер . – Л.: Химия, 1984. – 216 c.
- Кузнецов, Б. Н. Новые подходы в переработке твердого органического сырья / Б. Н. Кузнецов – Красноярск, 1991. – 371 с.
- Трепнел, Б. Хемосорбция / Б. Трепнел. – М.: Издательство иностранной литературы, 1958. – 326 с.
- Бур, Я. Х. Динамический характер адсорбентов / Я. Х. Бур – М.: Издательство иностранной литературы, 1962. – 291 с.
- Кельцев, Н. В. Основы адсорбционной техники / Н. В. Кельцев – М.: Химия, 1984. – 592 с.
- Гориславец, С. П. Пиролиз углеводородного сырья / С. П. Гориславец, Д. Н. Тменов, – Киев: Наукова думка, 1977. – 309 с.
- Мухин, В. М. Новые технологии получения активных углей из реактопластов / Мухин В. М., Зубова И. Д., Гурьянов В. В., Курилкин А. А., Гостев В. С.– 2009 - 195 с.
- А. О. Еремина, Углеродные адсорбенты из гидролизного лигнина для очистки сточных вод от органических примесей / А. О. Еремина, В. В. Головина, Н. В. Чесноков, Б. Н. Кузнецов - Journal of Siberian Federal University. Chemistry 1 (2011 4) С.100-107
- Манина Т. С. Получение и исследование высокопористых углеродных сорбентов на основе естественно окисленных углей кузбасса. Дис.к.х.н., Кемерово, 2013, - 133 с.
- Болдырев А. И. Физическая и коллоидная химия / А. И. Болдырев. - М., 1983.
- Кабачный В. И. Физическая и коллоидная химия / В. И. Кабачный, Л. К. Осипенко, Л. Д. Грицан и др. - Х., 1999.
