Abstract
List of content
- Introduction
- 1 Relevance
- 2 Measurement object analysis
- 2.1 Absorption and fluorescence spectrum of plant leaves
- 2.2 Measurement method
- 2.3 Formulation of the problem
- 3 Development of a structural diagram
- 4 Device layout
- References
Introduction
A fluorometer, or luminometric analyzer, is a device that allows you to determine the concentration of a substance by the level of the glow excited in them. The principle of operation of the fluorimeter is based on the phenomenon of fluorescence – the ability of molecules to emit light waves on their own, or do it after a chemical reaction with other substances. Knowing the luminescence of different compounds, it is possible to detect their presence in various compositions and substances.
Chlorophyll, the green pigment in plants, is directly involved in several physiological processes. By studying its fluorescence, we indirectly study other levels of photosynthesis, for example, processes at the level of pigments, reactions of electron movement, dark enzyme reactions, etc. [1]. These indicators are often decisive for the physiological state of plants. The manifestation of the influence of stress conditions in plants occurs at the stage when most of the physiological processes are already irreversible. Photosynthesis is the best indicator for determining plant health at the cell level. The fact is that the process of photosynthesis is often slowed down in plants, exposed to even short‑term adverse conditions – water scarcity, temperature, lack of nutrients, weed competition, penetration of pathogens [2, 3]. Therefore, analysis of chlorophyll fluorescence parameters is considered an important method for assessing health, the internal integrity of the plant within the aboveground part of the culture. It is also a quick and accurate way to diagnose and determine the degree of plant resistance to adverse or stressful conditions.
1 Relevance
At present, agriculture is actively developing on the territory of the Donetsk region and various types of crops are grown.
To grow quality products, it is often not enough to assess the condition of plants organoleptically. The plant's response to the influence of
stress factors (such as drought, excessive moisture, unacceptable pH level, etc.) in the form, for example, yellowed leaves appear too late for
prompt action to adjust the growing strategy. The most efficient of the currently known methods is the method of assessing the passage of
photosynthesis by measuring chlorophyll fluorescence [4]. This method involves the use of fluorimeter devices that irradiate
a plant leaf with a light flux, and then the response
of the plant is recorded, followed by the analysis of the recorded data, allowing to take
measures to promptly eliminate stress. Based on the foregoing, the study of the fluorometric method for assessing the state of plants is relevant.
In turn, to study this method, there is a need to develop a device capable of fixing the so‑called Kautsky curve,
the analysis of which will make it possible to determine the influence of stress factors on the plant.
The device under development for recording chlorophyll fluorescence in plant leaves will make it possible to determine how the plant feels under certain conditions, which will help to identify and eliminate factors detrimental to the plant, predict the trend in the development of the crop, simulate the maximum possible productivity of the crop in the existing conditions, etc.
2 Measurement object analysis
Chlorophyll fluorescence is not a very complex phenomenon. Its essence lies in the ability of the green pigment – chlorophyll – to absorb, and then emit (shine) light energy and long waves of light. The duration of such radiation by chlorophyll is determined by two factors: the amount of light absorbed by the plant and the level of competition for light energy with other energy processes, that occur in the plant (mainly, these are the processes of the formation of new substances and the transfer of heat in the plant). If chlorophyll begins to absorb less light, then its competitors receive more light energy. Hence, changes in the intensity of fluorescence reflect changes in the efficiency of the formation of new substances – our future harvest.
2.1 Absorption and fluorescence spectrum of plant leaves
ХChlorophyll is capable of selectively absorbing light. The absorption spectrum of a given compound is determined by its ability to absorb light of a certain wavelength (of a certain color). It has two main absorption lines in red and blue‑violet rays (Fig. 1). In this case, chlorophyll a has an absorption maximum at 429 and 660 nm, while chlorophyll b has an absorption maximum of 453 and 642 nm. However, one must consider that in a leaf the absorption spectra of chlorophyll change depending on its state, the degree of aggregation, adsorption on certain proteins [5]. It has now been shown that there are forms of chlorophyll that absorb light with wavelengths of 700, 710 and even 720 nm. These forms of chlorophyll, which absorb long wavelength light, are especially important in the process of photosynthesis.
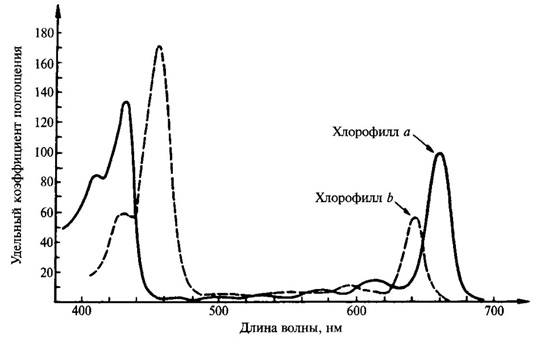
Figure 1 – Absorption spectrum of chlorophyll a and b
The pigment composition of the photosynthetic antenna complexes of higher plants is diverse. It includes 2 types of chlorophylls (Chl a, Chl b) and several types of carotenoids (lutein, violoxanthin, zeaxanthin, neoxanthin, β‑carotene), however, it is generally accepted that only Chl a molecules possess the ability to fluorescence under natural conditions, due to the fact that all the excitation energy received by other antenna pigments is transferred to the Chl a molecules [8]. The fluorescence spectrum of a green leaf is located in the red region of wavelengths (660 – 780 nm) and, as a rule, have two broad maxima (Fig. 2): one at 680 – 690 nm (F685), the other at 730 – 740 nm (F740) [8, 9]. The main contribution to the longwave the spectrum peak contributes to the reabsorption of the long‑wavelength forms of chlorophyll fluorescence emitted by the shorter‑wavelength forms [8, 9].
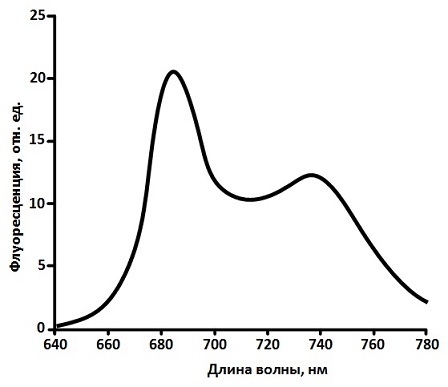
Figure 2 – The characteristic fluorescence spectrum of a green leaf
2.2 Measurement methods
The most practical and commonly used measurement methods are direct detection of fluorescence (long‑term chlorophyll induction [6]) and modulated fluorescence (light signal modulation method).
In the designed device, the first method is chosen as the measurement method, namely, direct registration of fluorescence (method of long‑term induction (stimulation) of chlorophyll). The choice of this method is justified by a comparative simple technical implementation, hence the greater cheapness relative to the second method.
Direct registration of chlorophyll fluorescence
The measurement is carried out after adaptation of the photosynthesizing object to the dark (about 20 – 30 min), which determines the attenuation
of the processes, associated with the light phase of photosynthesis. The sample is then illuminated with continuous light with a wavelength shorter than
670 nm. With the excitation light turned on, the photodetector records the chlorophyll fluorescence light in the wavelength range from about 680 nm to 760 nm.
By the nature of the fluorescence induction curve, one can judge some characteristics of the photosynthetic apparatus of the sample and the dynamics of
reactions of photosynthesis.
The described method is used in fluorimeters that measure the level of stress in a plant (the so‑called Plant Stress Meter). Such fluorimeters can record and analyze the fluorescence induction curves of chlorophyll for short times of exposure to active light, usually up to 10 seconds, but measurement should be carried out after dark adaptation of the plant
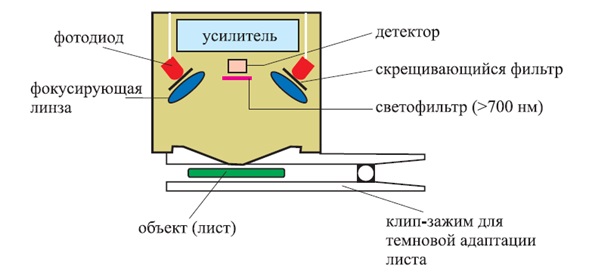
Figure 3 – Schematic of the measuring head of a fluorimeter using
When using this technique, the following parameters are determined:
- Fo – initial (zero) fluorescence;
- FM – maximum fluorescence level;
- Fv = FM - Fo – variable fluorescence;
- Fv / FM – maximum efficiency of ФС2;
- TFM – reach time FM;
- AM – surface area above the chlorophyll fluorescence induction curve.
2.3 Formulation of the problem
The aim of this work is to develop a device for recording chlorophyll fluorescence and its parameters in the leaves of the terrestrial parts of plants, capable of measuring directly in the places where these plants grow. If we consider modern devices fluorimeters as a prototype, the task can be called the development of the measuring unit of this device, but this “unit” is able to work independently, controlled with the help of special software from a PC, therefore it can be considered a complete device. This device, in contrast to potential prototypes, will have a significantly lower cost and simple design, which will make it affordable for ordinary users and small farms with a low budget. The main advantage of the developed device is relatively analogues – low price.
3 Development of a structural diagram
The device under development can be represented in the form of the following structural diagram:
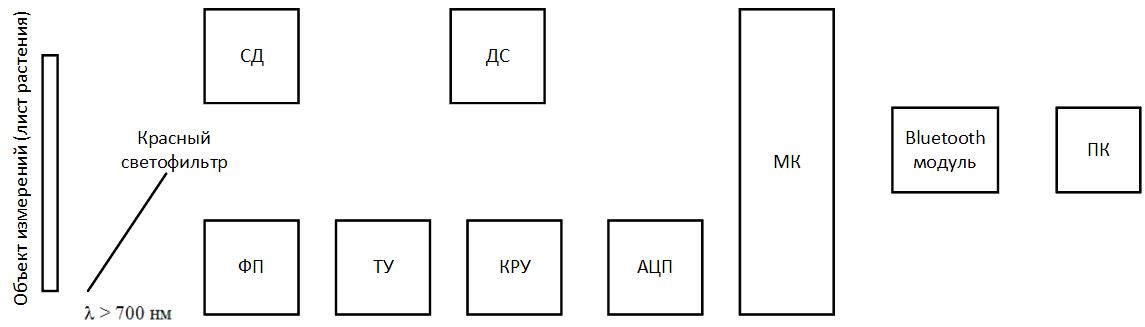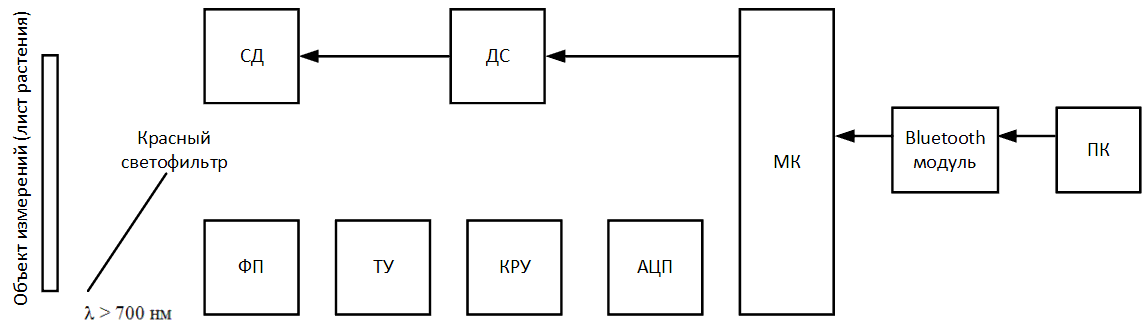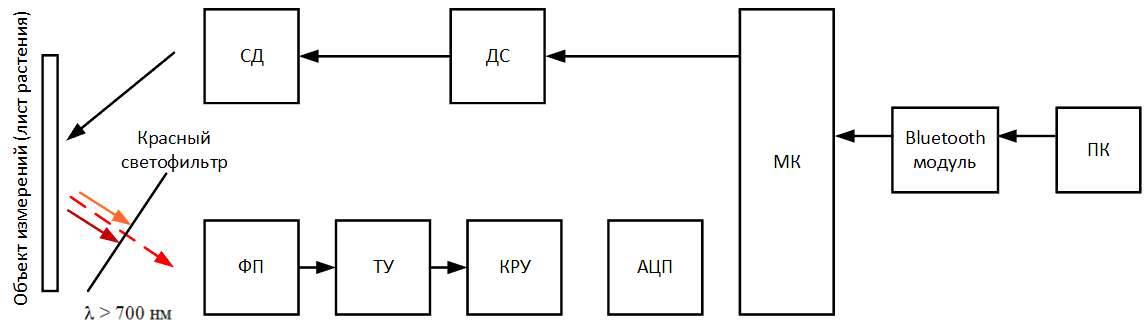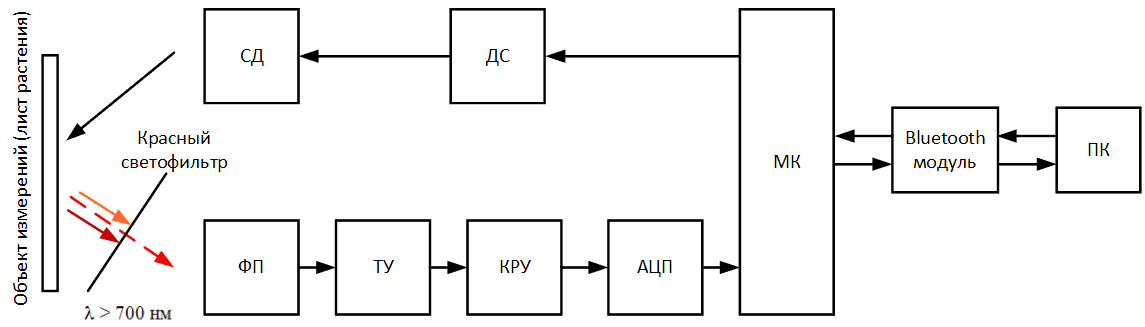
Figure 5 – Block diagram of the designed device (animated, 14 KB, 12 frames)
- ДС – LED driver;
- СД – LED;
- ФП – photodetector;
- ТУ – transimpedance amplifier;
- КРУ – gain control stage;
- АЦП – analog‑to‑digital converter;
- МК – microcontroller;
- ПК – Personal Computer.
The scheme has the following algorithm of work. In the developed software on a computer or laptop, the Start
button is pressed,
via the Bluetooth wireless network, a signal is sent to the microcontroller (МК) via a module built into the Bluetooth device, signaling the start of
measurement. The microcontroller sends an electrical signal to the LED driver (ДС), thereby lighting the LED (СД) and starting the process of lighting
the surface of the sheet plates. At the same time, the photodetector (ФП) is switched on and converts the fluorescence emitted by the sheet, which has
fallen on it through a red filter into electrical signal. The signal is amplified using a transimpedance amplifier (ТУ) and enters the gain control stage
(КРУ), in which it can be reduced or increased to a certain level, in order to avoid going beyond the measured range. The analog signal from the switchgear
goes to the analog‑digital a converter (АЦП), which converts an analog signal to digital and transfers it to a microcontroller (МК). MK throughout the
measurement retains measured fluorescence values (ADC code) into a special array, which at the end of the measurement is transmitted via the Bluetooth module
to a personal computer (ПК) for further processing in special software.
In accordance with the described algorithm, software was created in the object‑oriented programming language Delphi, presented in Figure 6.
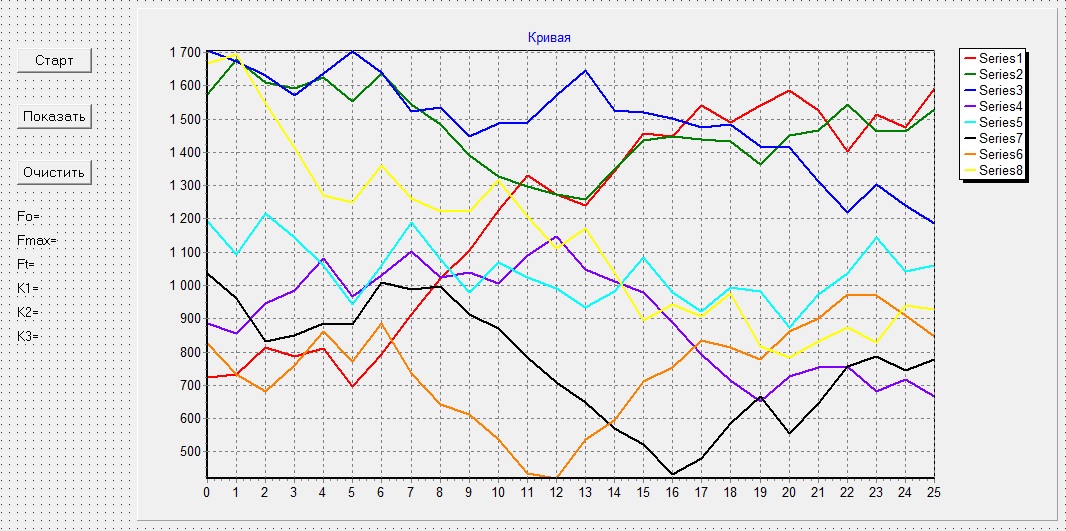
Figure 6 – PC software interface
4 Device layout
For testing the software and a more detailed practical study of the phenomenon of chlorophyll fluorescence, a prototype of the developed devices (fig. 7).
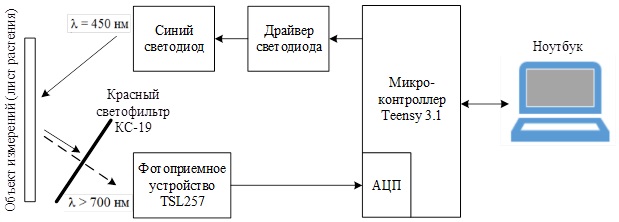
Figure 7 – Block diagram of the prototype of the device being developed
The principle of operation remains the same: a signal is received from the laptop to the microcontroller via USB, according to which the measurement begins, MK sends a signal to the blue LED driver, the LED irradiates the measurement object (plant leaf), while the leaf re‑radiates red fluorescence, which, through a red light filter, enters the photodetector, is converted into voltage and enters the ADC of the microcontroller, where it is digitized and stored in the operating memory of the MK. At the end of the measurement, the array of measured ADC codes is transferred via USB to a laptop, where it is processed in special software.
The dummy sample was tested on the leaves of different types of plants, and the resulting curves correspond to the curves, obtained by analogs of the device under development. One of the recorded curves is shown in Figure 8.
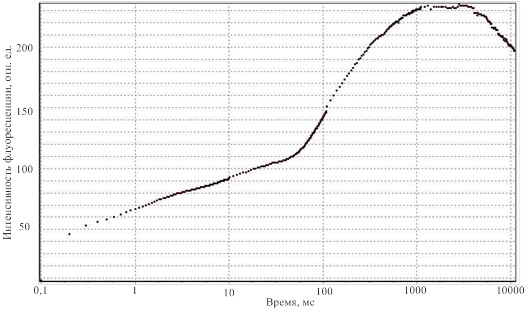
Figure 8 – The result of registering the Kautsky curve using the developed model of the designed device
At this stage, the device registers only the rapid fluorescence induction curve of chlorophyll, passing in the first 10 seconds of measurement. This allows you to get all the necessary parameters for determining the state of the plant, with the exception of FT, in the future the opportunity will be implemented carry out a complete measurement cycle and register all parameters.
References
- В. Н. Гольцев, М. Х. Каладжи, М. А. Кузманова, С. И. Аллахвердиев Переменная и замедленная флуоресценция хлорофилла a – теоретические основы и практическое приложение в исследовании растений. – М.–Ижевск: Институт компьютерных исследований, 2014. – 220 с.
- Л. А. Вадимович Механизмы токсического действия полициклическихароматических углеводородов на фотосинтетический аппарат, 2016.
- Д. А. Тодоренко Характеристики световых реакций фотосинтеза при воздействии токсических веществ, 2016.
- В. С. Лысенко, Т. В. Вардуни, В. Г. Сойер, В. П. Краснов Фундаментальные исследования, 2013, № 4–1. Флуоресценция хлорофилла растений как показатель экологического стресса: теоретические основы применения метода, 2013.
- Т. Э. Кулешова, А. И. Лихачев, Е. С. Павлова, Д. О. Кулешов, А. В. Нащекин, Н. Р. Галль Журнал технической физики, 2018, том 88, вып. 9. Взаимосвязь спектров поглощения пигментов растений и светодиодного освещения с различным спектральным составом
- Д. Ю. Корнеев Информационные возможности метода индукции флуоресценции хлорофилла, 2002.
- Г. В. Мельников, М. И. Лобачев, А. Г. Мельников Вестник СГТУ, 2003, № 1. Высокочувствительный импульсный флуориметр для экологического мониторинга окружающей среды
- C. Buschmann Variability and application of the chlorophyll fluorescence emission ratio red/far‑red of leaves // Photosynth. Res. 2007. V. 92. P. 261–271.
- К. Б. Асланиди, А. А. Шалапенок, В. Н. Карнаухов, Н. Г. Берестовская, В. И. Шавкин Метод определения функционального состояния растений по спектрам флуоресценции хлорофилла (техника биомониторинга). Пущино: НЦБИ АН СССР. 1988.
- N. A. Mir, R. Perez, R. M. Beaudry (1996) Chlorophyll fluorescence and whole fruit senescence in Golden Delicious apple. In: International Postharvest Science Conference Postharvest 96. – Taupo, New Zealand, p. 121–126.
- P. S. Basu, A. Sharma, N. P. Sukumaran (1998) Changes in net photosynthetic rate and chlorophyll fluorescence in potato leaves induced by water stress. Photosynthetica, 35:13–19.
- G. Angelini, P. Ragni, D. Esposito, P. Giardi, M. L. Pompili, R. Moscardelli, M. T. Giardi (2001) A device to study the effect of space radiation on photosynthetic organisms. Physica Medica, v. XVII, Supplement 1, 1st International Workshop on Space Radiation Research and 11th Annual NASA Space Radiation Health Investigators’ Workshop.
