Combustion synthesis of cobalt pigments: Blue and pink
T. Mimani and Samrat Ghosh
Department of Inorganic and Physical Chemistry, Indian Institute of
Science, Bangalore 560 012, India
http://www.ias.ac.in/currsci/apr102000/researchcommunication1.pdf
Idiochromatic blue cobalt aluminate (CoAl2O4) and purple pyroborate Co2B2O5 were prepared by solution combustion method using corresponding metal nitrates, boric acid and carbohydrazide mixtures. Allochromatic Co2+ doped in Al2O3/ZnAl2O4 and Mg2B2O5 pigments having the same colour intensity as idiochromatic pigments were obtained similarly. All the pigments are voluminous, homogeneously coloured with a large surface area. The products are characterized by their characteristic colours, XRD, IR and electronic spectra.
CERAMIC pigments are important in the industry due to their applications in tableware, sanitaryware, tiles and glasses. They can be classified into two broad categories: a) idiochromatic and b) allochromatic pigments. Idiochromatic (self-coloured) pigments are those in which transition metal ions are an essential part of the structure and contribute to the nature of the ligand field. Chrome green Cr2O3 and Thenard’s blue CoAl2O4 are some typical examples. Allochromatic (other coloured) pigments can be further sub-divided into two groups: a) substitution and b) inclusion type pigments. In substitution pigments, transition or lanthanide metal ions are present in the ligand field of the host lattice. For example, the pink colour of ruby (Cr3+/Al2O3) is due to trace amounts of chromium present in the octahedral site of the corundum lattice. Inclusion pigments are those in which coloured oxides are trapped or encapsulated in inert oxide hosts and can be considered as solid in solid type emulsion. The well-known red zircon (Fe3+: ZrSiO4) pigment is red due to the entrapment of Fe3+ ion within a zircon crystal.
The important physical–optical properties of pigments are their light absorption and scattering properties that depend upon the wavelength, particle size, particle shape and refractive index. Thus, for coloured pigments, the optimum particle size should be 1–10mμ and they should possess high refractive index to give good tinctorial strength (ability of a colourant to impart colour). Oxide materials like alumina, aluminates, silicates, borates, zirconia and zircons doped with transition metal or rare earth ions meet these requirements of ceramic colourants. Of these, the cobalt pigments are of paramount importance in ceramic industry due to their spectacular variety of colours, high tinting strength and remarkable stability under chemical, thermal and reducing conditions.
The co-ordination chemistry of cobalt complexes has fascinated chemists since Werner’s times due to the spectacular range of colours they produce. The various colours that these complexes are known to exhibit in solution are summarized in Table 1 (refs 4–6). It is interesting to note that the colours of cobalt (II) compounds are stereochemically specific depending upon the co-ordination site and number. While the octahedra cobalt compounds are generally pink to violet, the tetrahedral ones are blue. The production of different colours by cobalt compounds and ions is also possible in the solid state. Colours range from blue, green to pink depending upon the host lattice and co-ordination geometry. The conventional method for the preparation of these pigments is by the solid state reaction (ceramic method) using corresponding metal salts like oxides, carbonates, etc.7 This requires high temperature (1000–1400oC) and long processing time (several hours to days). The end products are usually coarse and inhomogeneous which render them inapplicable. They require further processing like wet or dry milling to produce fine powders. Addition of mineralizers is often necessary to facilitate the reaction in the fluid state.
Table 1. Various colours of Co(II) complexes
| Coordination compounds of cobalt (II) |
Colour |
Cobalt(II) coordination site |
| [Co(H2O)6]2+ |
Pink |
Octahedral |
| [Co(NH3)5Cl]2+ |
Pink |
Octahedral |
| [CoCl(H2O)5]+ |
Raspberry red |
Octahedral |
| Co(CH3COO)24H2O |
Violet red |
Octahedral |
| [Co(bipy)3]2+ |
Red |
Octahedral |
| CoF2 |
Pink |
Octahedral |
| CoCL2 |
Sky blue |
Octahedral |
| [CoCl4]2– |
Blue |
Tetrahedral |
| [Co(NCS)4]2– |
Blue |
Tetrahedral |
| [Co(N3)4)2- |
Blue |
Tetrahedral |
| [(C2H5O)2PS]Co(C2H5OH)2 |
Blue |
Tetrahedral |
| [Co(CN05]3- |
Dark green |
Tetragonal pyramidal |
The aim of this investigation is to prepare Co2+-based ceramic pigments by the solution combustion method. This method has been used successfully for the preparation of fine oxide materials. We intend to obtain the blue and pink colours of idiochromatic pigments such as CoAl2O4 and Co2B2O5 by doping minimum amount of Co2+ in Al2O3/ZnAl2O4 and Mg2B2O5 in order to make the process economical.
The combustible redox mixtures contain metal nitrates as oxidizers and carbohydrazide (CH)/urea as the fuel for the synthesis of ceramic pigments. A stoichiometric calculation of the redox mixtures is to be made to ensure complete combustion without any carbon residue. The Pyrex glass vessel containing aqueous solution of stoichiometric amounts of metal nitrates and fuel when introduced into a preheated muffle furnace (350oC–500oC) boils, froths, undergoes dehydration, ignites and burns to yield voluminous, foamy, homogeneously coloured pigments.
The spinel, cobalt aluminate (CoAl2O4) was obtained by the combustion of an aqueous solution containing 10 g Al(NO3)3•9H2O, 3.9 g Co(NO3)2, and 4.5 g CH. The solution, when rapidly heated at 350oC undergoes smoldering type combustion with intermittent sparks and flame (flame temperature ~ 1000oC) to give indigo-coloured pigment. The combustion yield is 13% giving 2.35 g of foamy cobalt aluminate (foam density = 0.015 g/cm3).
Cobalt doped zinc aluminate (CoxZn1–xAl2O4, x = 0.03) was prepared by the combustion of an aqueous solution containing 15 g Al(NO3)3•9H2O, 0.18 g Co(NO3)2, 1.6 g Zn(NO3)2 and 8 g urea. This solution was heated rapidly at 500oC. It undergoes flaming type combustion (flame temperature ~ 1350oC) yielding 4.8 g (12%) of foamy blue coloured cobalt doped zinc aluminate (foam density = 0.017 g/cm3). Similarly blue alumina (2.5 atom % of Co doping) was synthesized by rapidly heating aqueous solution containing 19.5 g Al(NO3)3•9H2O, 0.2 g Co(NO3)2•6H2O and 8 g urea at 500oC. The solution burns with a flame (flame temperature ~ 1500oC) yielding 10% (2.8 g) of foamy cobalt doped alumina (foam density = 0.009 g/cm3). Doping different atom % of Co produced various shades of blue alumina.
Cobalt pyroborate Co2B2O5 was prepared by rapidly heating (at 350oC) an aqueous solution containing 5 g Co(NO3)2•6H2O, 1 g H3BO3 and 2 g CH. The solution undergoes smoldering type combustion (flame temperature ~ 1000oC) to give a mauve pink (purple) product. The combustion yield is 24% giving 1.8 g of cobalt borate (foam density = 0.015 g/cm3). Rosy cobalt pigment was obtained by heating an aqueous solution containing 20 g Mg(NO3)2•6H2O, 1 g Co(NO3)2•6H2O, 4.8 g H3BO3 and 7.8 g CH rapidly at 350oC. The combustion is flaming type (flame temperature ~ 1250oC) yielding 17% (2.9 g) of rose pink Mg2B2O5 (foam density = 0.015 g/cm3). In all these cases the system is self-propagating going to the transient high flame temperature (1000o–1500oC). The reaction occurs within 10 sec with no damage to the pyrex glass vessel.
Combustion synthesized powders were characterized by X-ray powder diffraction patterns using a Shimadzu D-D1 Xray diffractometer with CuKa radiation and nickel filter. Infrared spectra were recorded using a Brucker FTIR Multiscan 15sf II instrument in a KBr matrix. The UV-Vis spectra for the powder samples were recorded in a diffuse reflectance mode at room temperature with Pye Unicam SP8-100 UV/Vis spectrophotometer using MgO as reference. Kubelka Munk equation was used to change the reflectance scale to the absorbance scale. The powder density was measured using a pyknometer with xylene as the liquid medium. The 50 wt% average agglomerate size was determined by sedimentation and light scattering principle using Micron photosizer instrument (model SKC 2000). BET surface area of the powders was measured by nitrogen adsorption using Accusorb Micrometrics instrument (Model 2100E).
Both allochromatic (CoxAl2–xO3 and CoxM1–xAl2O4) and idiochromatic (CoAl2O4) blue pigments were prepared as described above and characterized by their colour, XRD and UV-vis spectra. The theoretical equations for the formation of CoAl2O4 assuming complete combustion may be written as follows:
Co(NO
3)
3+2Al(NO
3)
3(aq) + 5CH
6N
4O(aq)=
CoAl
2O
4(s) + 5CO
2(g) + 14N
2(g) + 15H
2O(g).
The product is indigo in colour with a foamy texture (Figure 1). In a similar way, the equations for the formation of allochromatic blue pigments CoxAl2–xO3 and CoxM1–xAl2O4 (M = Mg and Zn, x = 0.01 to 0.2) can be represented as follows:
2Al(NO
3)
3(aq) + 5CH
4N
2O(aq)=
Co
x : α–Al
2–xO
3(s) + 5CO
2(g) + 8N
2(g) + 10H
2O(g)
3Zn(NO
3)
2(aq) + 6Al(NO
3)
3(aq) + 20CH
4N
2O(aq)
3Co
xZn
1–xAl
2O
4(s)+ 20CO
2(g)+ 32N
2(g) + 40H
2O(g)
Figure 1 a. CoAl2O1 and b. in Al2O3 foams
All the Co2+ (various proportions) doped Al2O3/ZnAl2O4 pigments are homogeneously blue in colour. The intensity of the blue colour gradually increases as the amount of Co2+ doping varies from 0.5 to 10 atom % in alumina as shown in Figure 2. At 5 atom % substitution the colour matches well to that of CoAl2O4. Hence the optimum concentration of dopant (Co2+) required to achieve the idiochromatic blue colour is between 5 and 7 atom % in alumina/ZnAl2O4 (Figure 3).
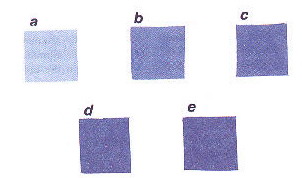
Figure 2 Variation of blue colour with Co2+ in Al2O3: a. 0.5 atom %;
b. 2.5 atom %; c.5 atom %; d. 7.5 atom %; e. 10 atom %.
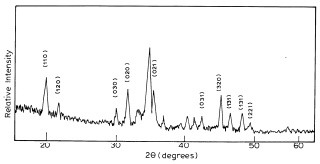
The combustion synthesized pigments are all crystalline as reflected from their XRD patterns. Figure 4 shows the diffraction pattern of the spinel CoAl2O4 and Co2+ doped in various proportions in hexagonal α-Al2O3 and the spinel ZnAl2O4. The X-ray pattern of 0.5 atom % Co2+ in Al2O3 shows a single phase of α-Al2O3. But as the substitution increases from 5 to 10 atom %, additional peaks start emerging. From 5 atom % onwards, CoAl2O4 phase starts appearing which becomes distinct at 10 atom %. The lattice parameters of the various blue aluminas refined with least square fit method show a gradual decrease in the ‘a’ and ‘c’ values when compared to the unsubstituted one. For a Co2+ substitution of x = 0.01 to 0.1, ‘a’ values change from 0.4772 nm to 0.4767 nm and ‘c’ values change from 1.3043 nm to 1.3013 nm. The increase in the cobalt content causes a decrease in the lattice constant, indicating an increase in the crystal field around the Co2+ ion. This could also be the reason for the deepening of the blue colour with an increase in cobalt doping together with the increase in CoAl2O4. After 7.5 atom % the change in the lattice parameters shows some discrepancy. Although the shrinkage in the ‘c’ parameter continues, the ‘a’ parameter elongates. It appears that the formation of the spinel CoAl2O4 which increases thereafter (Figure 4), begins to influence the hexagonal lattice of Al2O3, making it more cubic (a = 0.8103 nm).
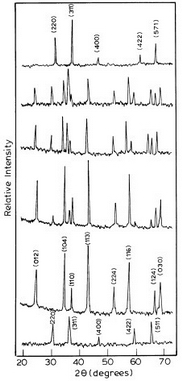
Figure4 Powder XRD patterns of (a) CoAl2O4;(b) 0.5 atom %; (c) 2.5 atom %;
(d) 7.5 atom %; (e) 10 atom % of Co 2+:Al2
O3; (f) 2.5 atom % of Co 2+: ZnAl2O4
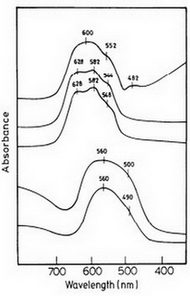
The diffuse reflectance spectra of CoAl2O4, CoxAl2–xO3 and CoxZn1–xAl2O4 are shown in Figure 5. The electronic spectra of combustion synthesized cobalt alumina blue pigments are characterized by a broad absorption band whose centroid is placed in the red part of the visible spectrum. The intense convoluted absorption band with prominent absorbance peaks at around 550, 580 and 620 nm indicates tetrahedral co-ordination of Co2+. From Tanabe Sugano energy level diagram the spin allowed Laporte forbidden d–d transitions of Co2+ (d7 system) in tetrahedral coordination are:
≤1: 4A2(F)(e4t2
3) → 4T2(F)(e3t2
4)
≤1:
4A2(F) → 4T1(F)(e3t2
4)
≤3:
4A2(F) → 4T1(P)(e2t2
5)
where ≤1 < ≤1 < ≤3.
Figure 5. Diffuse reflectance spectra of (a) CoAl2O4; (b) Co2+ :
aAl2O3;
(c) Co2+ : ZnAl2O4; (d) Co2B2O5 and (e) Co2+ : Mg2B2O5.
The wide and intense absorption band between 500 and 700 nm could be attributed to the third transition (≤3) from 4A2(F) ground state to the excited 4T1(P) state. The fine spectra which is diagnostic of tetrahedrally co-ordinated Co2+ arises due to spin orbit coupling between the excited and the neighbouring doublet states 2A1(G) and 2T2(G). Because of mixing of quartet and doublet states, the spin selection rule is relaxed and the transition is allowed. The other two transitions (≤1 and ≤2) are not observed as they lie in the far infrared region.
4A2(F) → 2A1(G) and 2T2(G)
The spectrum is similar in all the three samples and arises due to the presence of cobalt in the tetrahedral site of the spinel CoAl2O4. The a-Al2O3 lattice has a hexagonal corundum structure wherein the substitution of Co2+ in place of Al3+ cannot take place due to the larger size of Co2+. It therefore occupies the interstitials as CoAl2O4 as reportedearlier and evident from the XRD patterns which show this spinel phase. The solid solution formed here is an inclusion type. However, in the case of ZnAl2O4 matrix, the substitution of Co2+ in the place of Zn takes place to impart the colour. The solid solution is substitution type in the spinel ZnAl2O4
Similarly, allochromatic (CoxMg2–xB2O5) and idiochromatic (Co2B2O5) mauve-pink (purple) pigments have been prepared as described above and characterized by their colour, XRD, IR and UV-visible spectra. The theoretical equations assuming complete combustion for the formation of Co2B2O5 and CoxMg2–xB2O5 can be written as:
4Co(NO3)2(aq) + 4H3BO3(aq) + 5CH6N2O(aq) →
2Co2B2O5(g) + 5CO2(g) + 14N2(g) + 21H2O(g)
4Mg(NO3)2(aq) + 4H3BO3(aq) + 5CH6N2O(aq) →
2(CoxMg2–xB2O5)(s) + 5CO2(g) + 14N2(g) + 21H2O(g)
The colour of the allochromatic CoxMg2–xB2O5 compares well with the purple colour of Co2B2O5 as shown in Figure 6 (5 atom % of Co doping). The as-synthesized Co2B2O5 is X-ray amorphous. The crystalline phase occurred after sintering at 1200oC for 4 h. Powder XRD patterns of Co2B2O5 and CoxMg2–xB2O7 are similar to triclinic Mg2B2O5 (Figure 7). The structure of magnesium pyroborate could be described as constructed of 2-D layers formed by the pyroborate (B2O54–) groups. The composite ion B2O54– is formed by two BO33– triangles linked by one oxygen atom. The connection between the groups in the intra and inter layers is maintained by Mg2+ ions which are surrounded by 6 oxygen atoms forming a deformed octahedron. The strong bands at 1200, 700 and 600 cm–1 in the FT-IR spectra indicate the existence of BO3 groups in the orthoborate crystal structure.
Figure 6 Comparison of (a) Co2B2O5 colour with (b) 5 atom % of Co2+ in Mg2B2O5
Figure7 Powder XRD patterns of as-prepared Co2+ in Mg2B2O5
The reflectance spectra of Co2B2O5 and CoxMg2–xB2O7 pigments are presented in Figure 5. The spectrum of CoxMg2–xB2O7 is similar to that of violet-red Co(CH3COO)2•4H2O in which cobalt is octahedrally coordinated by acetate ligands. Octahedral cobalt compounds are generally pink in colour. Thus in rose-pink CoxMg2–xB2O7, cobalt substitutes for magnesium in the octahedral lattice site of the pyroborate structure. The purple Co2B2O5 shows a similar but a broader electronic spectrum suggesting cobalt in an octahedral environment. The band at around 560 nm for both Co2B2O5 and CoxMg2–xB2O7 could allowed Laporte forbidden d–d transition.
4T1g(F)(t2g5eg2) → 4T2g(P)(t2g4eg3).
The particulate properties such as apparent powder densities, specific surface area, average agglomerate size of these pigments are summarized in Table 2. The fine nature of the pigments is evident from the tabulated data. The exothermicity of metal nitrate–urea reaction (> 1500oC) is quite high for the preparation of CoAl2O4 and borates which are unstable above 1000oC. Therefore, it was necessary to use hydrazide fuel that undergoes smouldering (flameless) combustion with metal nitrate. This method was further used to synthesize various colours of Co2+-based allochromatic and idiochromatic pigments as shown in Table 3.
Table 2. Particulate properties of the various pigments
| Pigments |
Fuel |
Flame temp.oC |
Crystallite size from XRD (nm) |
Powder density |
BET surface area (m2/g) |
Particle size from surface area (µm) |
Average agglomerate size (µm) |
CoAl2O4
(Indigo) |
CH |
1100 |
18 |
2.32(53) |
58 |
0.100 |
4.5 |
CoxAl2–xO3
(Blue) |
Urea |
1500 |
40 |
2.75(70) |
10 |
0.220 |
4.0 |
Co2B2O5
(Purple) |
CH |
950 |
- |
1.85 |
12 |
0.27 |
2.5 |
CoxMg2–xB2O5
(Pink) |
CH |
1350 |
15 |
2.1(72) |
8 |
0.35 |
2.2 |
Table 3. Combustion synthesized cobalt (II) pigments
| Pigments (Crystal class) |
Colour |
Cobalt (II) co-ordination site |
Co2+:ZnB4O7
(Metaborate) |
Violet |
Tetrahedral |
CoAl2O4
(Spinel) |
Indigo |
Tetrahedral |
Co2+:ZnAl2O4
(Spinel) |
Blue |
Tetrahedral |
Co2+:Zn2SiO4
(Phenacite) |
Lavender |
Tetrahedral |
CoCr2O4
(Spinel) |
Blue-green |
Tetrahedral |
Co2+:ZnO
(Wurtzite) |
Green |
Tetrahedral |
Co2+:Mg2B2O5
(Pyroborate) |
Rose-pink |
Octahedral |
Co2B2O5
(Pyroborate) |
Purple |
Octahedral |
The objectives that have been achieved through these investigations are as follows:
- Co2+-based blue and pink allochromatic pigments matching the idiochromatic pigment colours of CoAl2O4 and Co2B2O5 have been prepared successfully by the solution combustion process;
- Optimum amount of Co2+ ions required to achieve the colour intensity of idiochromatic pigments has been determined (e.g.5 atom % Co2+ in Al2O3/ZnAlO4/Mg2B2O5);
- The allochromatic pigments can be made using a cheaper fuel like urea with less amount of the colouring atom, so that manufacturing cost is reduced;
- The solution combustion process described for the synthesis of pigments is simple, fast and energetically attractive. It has the advantage of rendering homogeneity, purity and molecular level doping of Co2+ (colourant) in the oxide matrices;
- As the pigments are fine with large surface area, one needs smaller amount of these pigments to coat a given surface of the ceramic body;
- The process is a ‘single step process’and so can be scaled up easily.
Literature
- Evans, W. D. J., Trans. J. Br. Ceram. Soc., 1968, 67, 397.
- Eppler, R. A., in Ullmann’s Encyclopedia of Industrial Chemistry, VCH Publishers Inc., Weinheim, West Germany, 1986, vol.
A5, p. 163.
- DeBie, E. and Doyen, P., Cobalt, 1962, 15, 3–13; 16, 3–15.
- Greenwood, N. N. and Earnshaw, A., Chemistry of Elements,
Pergamon Press, 1989, pp. 1312–1313.
- Bjerrum, J., Coord. Chem. Rev., 1989, 94, 1.
- Cotton, F. A. and Goodgame, M., J. Am. Chem. Soc., 1961, 83,4157 and 4690.
- Richard, A. E., Ceram. Bull., 1987, 66, 1600.
- Patil, K. C., Aruna, S. T. and Ekambaram, S., Curr. Opin. Solid State Mater. Sci., 1997, 2, 158.
- Suresh, K. and Patil, K. C., in Perspectives in Solid State Chemistry (ed. Rao, K. J.), Narosa Publishing House, New Delhi, 1995,p. 376.
- Feng Ren, Shingo Ishida, Nobuyuki Takeuchi and Kaichi Fujiyoshi, Ceram. Bull., 1992, 71, 759.
- Alarcon, J., Escribano, P. and Marin, R. M., Br. Ceram. Trans. J., 1985, 84, 17.
- Sutton, D., Electronic Spectra of Transition Metals, Reverte,Barcelona, 1975.
- Carlin, R. L., Transition Metal Chemistry, 1965, vol. 1, pp. 1–33.
- Nelson, C. and White, W. B., J. Mater. Res., 1986, 1, 130.
- Stanley Block, J. Res. Natl. Bur. Stand., 1959, 62, 95.
- Cotton, F. A. and Willkinson, G., Advanced Inorganic Chemistry, A Comprehensive Text, John Wiley, New York, 1980, 4th edn.