Abstract on the Master`s work
Contents
Introduction
1.Analytical review of the methods of processing acid tars
2.Investigation of physical and chemical composition of pond acid tars
3.The study of the dewatering process pond acid tars
Conclusion
References
Introduction
In the process of production of petroleum products, including distillates, motor oil and other oils are widely used methods of treatment associated with the use of concentrated sulfuric acid or oleum [1]. This removes the unsaturated and aromatic hydrocarbons, as well as sulfur and nitrogen compounds, resinous substances, which reduce the stability and performance of commercial petroleum oils [2,3].
The use of sulfuric acid method of treatment is accompanied by significant losses of products undergoing polymerization or dissolved in acid, as well as the formation of hard recyclable waste – acid tars.
Acid tars, which are stored in the impoundment (Fig. 1), are a source of pollution. So is the search for new methods of treatment that would give up the sulfuric acid method. The composition of pond acid tars (PAT) is strongly depends on feedstock and the processing conditions.

Figure 1 – Acid tar pond Mittelbahe, Germany
ПStorage pond of acid tars are located near all the major refineries (as previously sulfuric acid method of cleaning oil used almost everywhere). These ponds can be found in the U.S.A., Belgium, Germany, Latvia, Russia, China, Ukraine, etc. [4]. Ponds drives threaten ecological disaster for region, projects are developed for the disposal pond acid tars and restoration of contaminated lands in recent years.
Pond acid tars – man-made resources, which are the source of hydrocarbons, which is obtained from non-renewable resource – oil.
The aim is to provide a method for utilization of PAT. To achieve this goal it is necessary:
1 Analytical review of the methods of processing acid tars
At present there is no universal method of processing of PAT, because their composition is very diverse. There are many ways of processing acid tars offered at the moment . They are divided into four groups:
The best of these is the fourth group - complex processing acid tars. And while each method has both advantages and disadvantages. Therefore a combined method of processing is more likely to use.
2 Investigation of physical and chemical composition of pond acid tars
Samples of acid tars from different ponds were taken for study in the laboratory of CTF to develop a processing technology tars in commercial products.
The composition of PAT is complex and not well understood. It is known that they are a multi-component mixture of resins, oils, asphaltenes, condensed heteroaromatic compounds, sulfonic acids, sulfuric acid, acid and sulfuric acid esters of medium-sized, water and mineral impurities. Such complex systems are characterized by their content of group components. Group composition of PAT is determined on the basis of different solubility in organic solvents components – gasoline and toluene [6]. Let’s consider the properties of group components of PAT.
Oils and resins. Elemental composition of oils is as follows: carbon (85 ÷ 88) %, hydrogen (10 ÷ 14) %, sulfur less than 4,5 % and a small amount of oxygen and nitrogen. The molecular weight of oils (240 ÷ 800) (usually 360 ÷ 500), the ratio C:H (atomic), which characterizes the degree of aromaticity, is usually equal to (0,55 ÷ 0,66). The density of oils is less than 1 g/cm3.
Resins at normal temperature – - are solids substances reddish-brown color. Their density is (0,99 ÷ 1,08) g/cm3. They belong to the high-molecular organic compounds with heterocyclic and cyclic structure with a high degree of condensation, connected together by aliphatic chains. They include the addition of carbon (79 ÷ 87 %) and hydrogen (8,5 ÷ 9,5 %) oxygen (1 ÷ 10 %), sulfur (1 ÷ 10 %), nitrogen (2 %) and plenty of other elements including metals (Fe, Ni, V, Cr, Mg, Co, etc.). The molecular weight of resins (300 ÷ 2500).
Asphaltenes. Asphaltenes are considered as the product of resins’ condensation. In the free form, they are solid non-consumable brittle materials black or brown in color. Asphaltenes are extracted from crude oils and heavy oil residues by deposition from solutions of mineral oil in a 40-fold volume of petroleum ether, n-pentane, isopentane, or 10-fold volume of n-heptane.
Share and composition of isolated asphaltenes depend on the solvent used and the deposition conditions. The density of asphaltenes more than 1 g/cm3. ЭElemental composition (in wt.%): carbon (80 ÷ 84); hydrogen (7,5 ÷ 8,5); sulfur (4,6 ÷ 8,3); oxygen less than 6, nitrogen (0,4 ÷ 1).
The chemical composition of asphaltenes are not well understood. Several types of polycyclic structures are proposed as the main links of the molecules of resins and asphaltenes. The most likely structure of asphaltenes – is (12 ÷ 14) condensed rings with alternating aliphatic side chains and oxygen atoms or sulfur atoms in these chains or rings. The following structure of asphaltenes is likely also (1):

The structure of asphaltenes can also be represented by four equal quad-groups linked to each other by heteroatoms (2). Each group contains two aromatic and two naphthenic nucleus (arrows indicate the location in which easily carried out condensation):

The ratio of C: H (atomic) for the asphaltenes is in the range (0,94 ÷ 1,3); the degree of aromaticity is (2,8 ÷ 4,7).
Asphalt acids. Asphalt acids and their anhydrides – brown-gray materials with thick resinous consistency. Asphalt acids are readily soluble in alcohol or chloroform and difficult – in gasoline and a density of them more than 1 g/cm3.
Carbenes and carboids. Carbenes and carboids are high carbon products of high-temperature processing oil and its residues. Carbenes are insoluble in carbon tetrachloride, carboids – in carbon disulfide [7].
Determination of water in the samples of tar was performed according to the Dean-Stark method, its equipment is shown in Figure 2 [8].

1 – round-bottomed flask; 2 – the Dean-Stark’s nozzle; 3 – reflux condenser.
Figure 2 – Equipment of the Dean-Stark method
This table presents the results of chemical analysis of samples.
Table – Characteristics of pond acid tars
| Group composition, % | Acidity H2SO4, % | H2O, % | Ad, % | S, % | Oils + resins | Asphaltenes | Carbenes + carboids | Pond 1 Sample 1 |
7,7 | 39,2 | 53,1 | - | 19,6 | 5,0 | 3,7 | Sample 2 | 92,1 | 4,1 | 3,8 | 0,5 | 33,3 | 1,8 | 0,65 | Sample 3 | 11,1 | 61,1 | 27,8 | 0,8 | 34,6 | 6,0 | 1,7 | Pond 2 Sample 4 |
63,3 | 17,3 | 19,4 | 0,6 | 39,2 | 2,5 | 2,3 | Pond 3 Sample 5 |
86,6 | 8,6 | 4,8 | 0,2 | 45,6 | 2,0 | 1,8 |
As can be seen from the data of the three acid tars ponds are characterized by high moisture content (33,3 ÷ 45,6 %), the acidity in terms of sulfuric acid is less than 1 %. Samples taken from different depths of the pond number 1 differ by group composition, ash content and sulfur. The upper layer (sample number 1) contains a minimal amount of oils and resins 7.7 % and the maximum number of carbenes and carboids 53.1 % and 3.7 % sulfur. The sample number 2 (with a depth up to 1 m) contains the maximum number of resins and oils 92.1 % and the minimum amount of asphaltenes 4.1 %, carbenes and carboids 3.8 %, lower in this sample of ash and sulfur (respectively 1.8 % and 0.65 %). With increasing depth of sampling up to 1.5 m (sample number 3) is increased asphaltenes content 61.1 % and 6.0 % ash.
The sample number 4 (pond number 2) contains resins and oils 63.3 %, 17.3 % asphaltenes, carbenes and carboids 19.4 %. The sample number 5 (pond number 3, depth to 3.5 m) resins and oils are more than 86.6 % and less than 8.6 % of asphaltenes and carbenes with carboids are 4.6%. Ash and sulfur content for the two ponds are respectively (2,0 ÷ 2,5) % and (1,8 ÷ 2,3) % [6].
High humidity of PAT makes it necessary first of all their dehydration. And the presence of acid requires neutralization.
Based on the fact that the composition of PAT is different from each pond can produce different commercial products (depending on which component is predominant in them). This is graphically represented in a diagram in Fig. 3 [7].
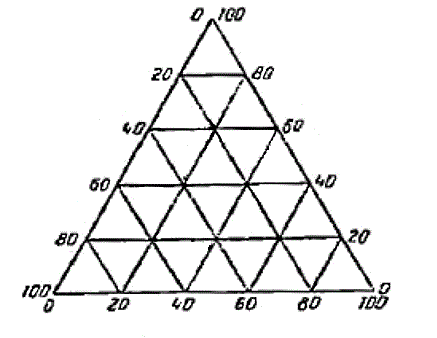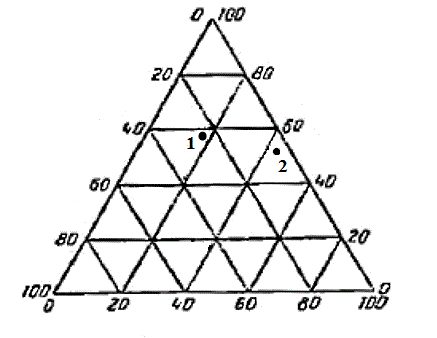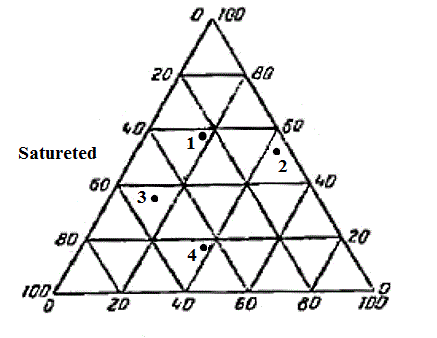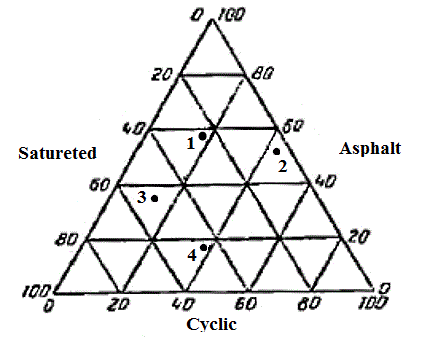
1 – typical roofing; 2 – coal-tar pitch; 3 – road; 4 – the basis of bitumen emulsion
Figure 3 – The triangular diagram of group composition different bitumen (wt %)
Saturated components in this case includ paraffin- naphthenic hydrocarbons, to cyclic – aromatic hydrocarbons, and to the asphalt – asphaltenes and asphaltic resins. For roofing bitumen, for example, the content of saturated hydrocarbons in the range (23,8 ÷ 55) wt. % and cyclic compounds (11,8 ÷ 33,9) wt. %. The content of the first falls under the natural weathering of bitumen, which accelerates the aging process. This fact must be considered when selecting the optimal composition of roofing bitumen.
3 The study of the dewatering process pond acid tars
The first step in processing acid tars is their dehydration. It was found that the gravitational settling at a temperature of (80 ÷ 90) оC is inefficient, cause in this case it is possible to allocate (2 ÷ 4) % of water. Therefore, the removal of moisture from the acid tars were carried out by evaporation. A portion of acid tar was placed in a reactor and heated at a temperature of (95 ÷ 115) оC, 0С stirring constantly. The results show that: stirring for (2 ÷ 4) hours shows the degree of dehydration of acid tars amounted to (98 ÷ 100) %. The residual amount of water ranges from 0 % to 1 %.
Dehydration of acid tars changes group composition – content of asphaltenes increases and content of resins, oils, carbenes and carboids decreases. This is a result of the reactions of polycondensation and polymerization. The highest reactivity of the sample showed the number 4 from the pond number 2 – resins and oils in it decreased from 63.3 % to 14.2 %, and the amount of asphaltene increased from 17.3 % to 66.1 %. Dehydrated sample number 4 (pond number 2) and number 3 (pond number 1) is a hard brittle material at room temperature. Acid tars (sample number 2 and number 5) after the removal of water – it is homogeneous viscous resinous mass [6].
With the presented results we can assume some bituminous product may be obtained from the corresponding samples. Of the samples number 2 and number 5 – liquid fuel (because their composition is dominated by oil, which provides them with a liquid state under normal conditions). A sample of the № 1, № 3 and № 4 after further processing and adding a modifier – bitumen.
Completed studies have shown that in developing a method of processing a PAT in a variety of commercial products must take into account complex physical and chemical processes occurring during the dehydration of tar.
Conclusion
Pond acid tars are a waste oil processing, non-renewable natural resource. They pollute the environment, occupy large areas and threaten the ecological disaster for regions. At the same time, the PAT can be a source of hydrocarbons for the production of various commercial products.
Analytical review of the methods of processing PAT has shown that there is no universal method suitable for the processing of tar of any composition at present.
The composition of the PAT was investigated and it showed that the content of the main components varies widely:
A dehydration of PAT is carried by distilling off the water at a temperature of (95 ÷ 115) оC. The possibility of dehydration in this way is showed to the achievement of the degree of dehydration of (98 ÷ 100) %. An acidity of the reaction mixture decreases as a result of dehydration.
It is established that it is possible to obtain commercial products (mostly asphalt) from the PAT, but this should be undertaken:
Master's work is not yet completed in writing this abstract. Completion date of work – December 2012. Full text of the work and materials on this topic can be obtained from the author or his head after that date.
References
-
Абросимов А.А. Экология переработки углеводородных систем. М.: Химия, 2002. – 468-475 c.
Фролов А.Ф., Титова Т.С., Карпова И.В., Денисова Т.Л. О составе кислых гудронов сернокислотной очистки нефтяных масел // Химия и технология топлив и масел. 1985. – № 6. – С. 37-38.
Крейцер Г.Д. Асфальты, битумы, пеки. М.: Промстройиздат, 1952. – 1734 c.
Серно кислые гудроновые озера и методы их утилизации [an electronic resource]. – Access mode: http://www.corvus.lv
Шухов В.И., Тишакова А.Н. Кислые гудроны и проблемы их утилизации [an electronic resource]. – Access mode: http://www.techros.ru/
Бонадрев А.С., Колбаса В.А., Крутько И.Г. Изменение физико-химического состава прудовых кислых гудронов при обезвоживании. Охрана окружающей среды и рациональное использование природных ресурсов: сборник докладов Всеукраинской конференции аспирантов и студентов. – Донецк: ДонНТУ, ДонНУ, 17-19 апреля 2012. – Т.2. – С. 188-189.
Гун Р.Б. Нефтяные битумы. – М.: Химия, 1973. – С. 9–13.
Большая Энциклопедия Нефти и Газа. [an electronic resource]. – Access mode:
http://www.ngpedia.ru
