Abstract
Content
- Introduction
- 1. Modern condition of a question
- 2. Description of the scientific results of the master's work
- Conclusion
- References
Introduction
Master's work is devoted to investigation of the processes of convective mass transfer for hydrogen furnace-ladle degassing of the melt copper complex influence of vacuum and distributed on the bottom of the bucket argon blowing, as well as development of energy-saving modes of this technology. The problem of energy saving in Ukraine - one of the most actual.
1. Modern condition of a question
To obtain dense copper ingots to reduce the amount of hydrogen in the melt, because otherwise, during crystallization formed pores. One of the most promising ways to remove hydrogen from copper - integrated impact vacuum and blowing with argon. Into force of the diffusion of hydrogen in liquid copper, this comprehensive treatment of minimally [1]. In accordance with the well-known mechanism of degassing of the melt copper dissolved hydrogen [2], the process of mass transfer in the liquid bath includes the following stages:
- transfer by convection hydrogen atoms to interface surfaces "argon-metal" and "vacuum-metal";
- transport of these particles through a diffusion boundary layers considered borders;
- adsorption of hydrogen atoms at these borders;
- chemical reaction mole them atoms in a molecule of hydrogen;
- diversion of molecules of hydrogen bubbles argon and vacuum chamber.
Degassing action vacuum and blowing melt metal with argon occurs when there is a difference of concentrations of hydrogen in liquid bath and at interfaces surfaces «vacuum-metal» and «argon-metal» [2]. With increasing intensity of purging efficiency technologies, as a rule, increases. However, a detailed investigation of this dependence shows that it is more complicated [3-10]
2. Description of the scientific results of the master's work
Computer simulation technology allows you to explore it using different parameters: the height of the liquid bath and pore diameter purge devices, the intensity of argon blowing, the pressure in the vacuum chamber, the concentration of surface-active elements (AEO) in the melt copper and others.
The main results of computer simulation during degassing of the melt copper from the hydrogen in the functions of the technological parameters is shown in figure 1-5.
In Fig.1 the technological parameter is the intensity of the scattered by porous bottom of the bucket purge melt with argon. As follows from this chart, fixed-time processing with increasing intensity level of degassing of liquid metal rises, but to a level equal to 0.04 m3/s, and then decreases. This is explained by the increase in the number of the bubbles argon introduced into liquid steel per unit of time, with increasing intensity. However, as known from the experiment, refine ability bubbles argon decreases with increasing intensity blowing, and that leads to the presence of a maximum in the dependence of the degree of degassing. At the intensity equal to 0 degassing of the melt copper occurs due to the vacuum, the depth of which determines the efficiency of complex processing of copper.
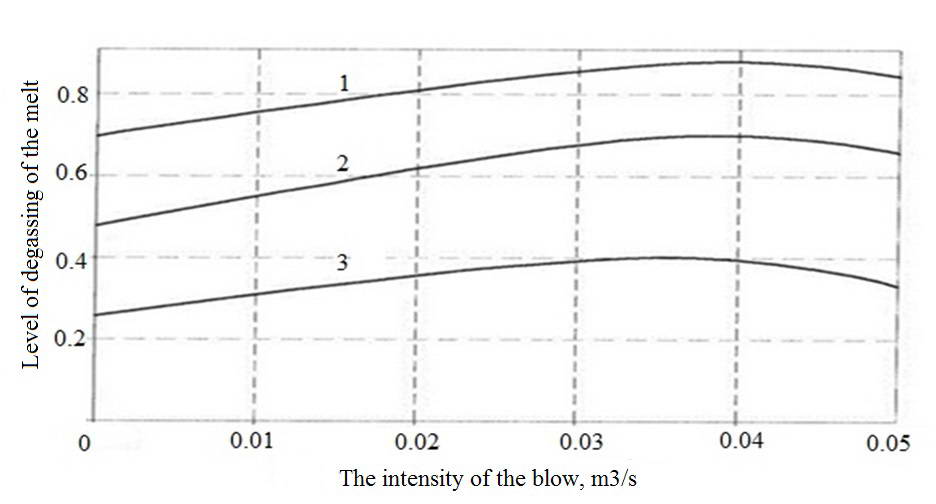
Fig. 1. The dependence of the degree of degassing of the melt copper on the intensity of argon blowing
1 - 600 PA; 2 - 400 PA; 3 - 200 PA
Graph of dependence of the degree of degassing of metal purge of the device is shown in figure 2. the intensity of argon blowing fixed. Decreasing the nature of the dependence is explained by the increase in the area of interphase surface "argon-metal" and , respectively, the intensity of diffusion mass transfer with a decrease of the pore diameter. Increasing the degree of rarefaction vacuum chamber leads to the increase of the degree of degassing of the melt.
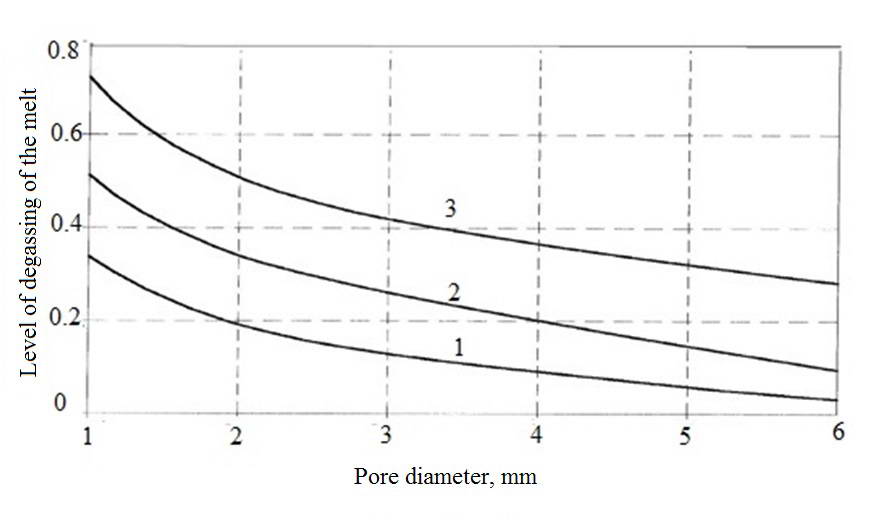
Fig. 2. The dependence of the degree of degassing of the melt copper from the pore diameter blow-off device
1 - 600 PA; 2 - 400 PA; 3 - 200 PA
Figure 3 illustrates the schedule of dependence of ? concentration of surface-active elements, in particular, the oxygen contained in the melt in dissolved form and in the form of oxide inclusions. The increase in the effective concentration of AEO leads to more blocking of inter-phase surfaces "argon-metal" and "vacuum-metal" these elements, and to slow the diffusion of hydrogen on these surfaces.
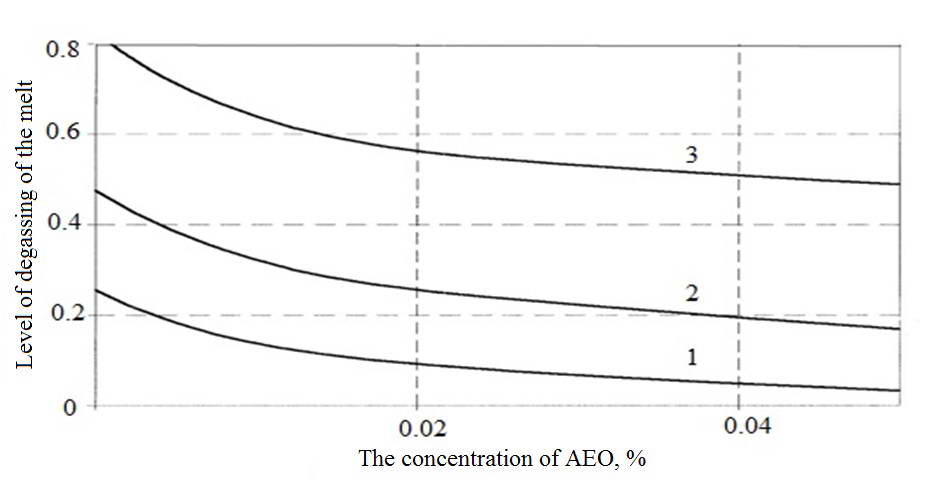
Fig. 3. The dependence of the degree of degassing of the melt copper concentration of surface-active elements
1 - 600 PA; 2 - 400 PA; 3 - 200 PA
On Fig. 4 shows the dependence of the degree of degassing of the melt copper from the depth of the liquid bath. A growing part of this dependence is explained by an increase absorptive bubbles argon at increasing the length of their path of ascension. However, in the final sections of this path bubbles argon are saturated with hydrogen and large depth of liquid bath of these areas they are in the mode of idling, which causes the output graph curve to the maximum.
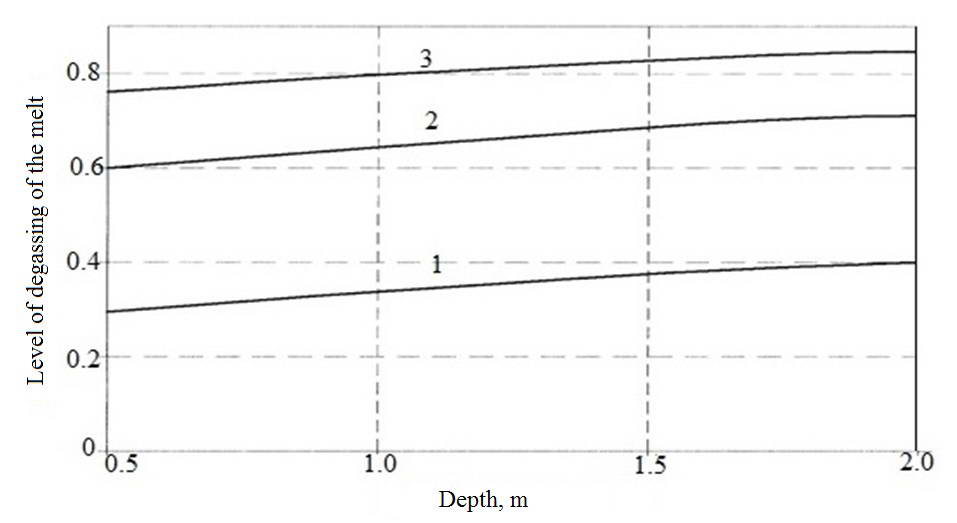
Fig. 4. The dependence of the degree of degassing of the melt copper from the depth of the bath
1 - 600 PA; 2 - 400 PA; 3 - 200 PA
The graph (Fig. 5) reflects the contribution during degassing purge copper argon in the overall degree of its degassing of hydrogen. As follows from this chart, with significant intensity dispersed by porous bottom of the bucket melt blowing argon, its contribution to the effectiveness of complex processing of copper significantly higher vacuum, especially, with not very high degree of rarefaction vacuum chamber
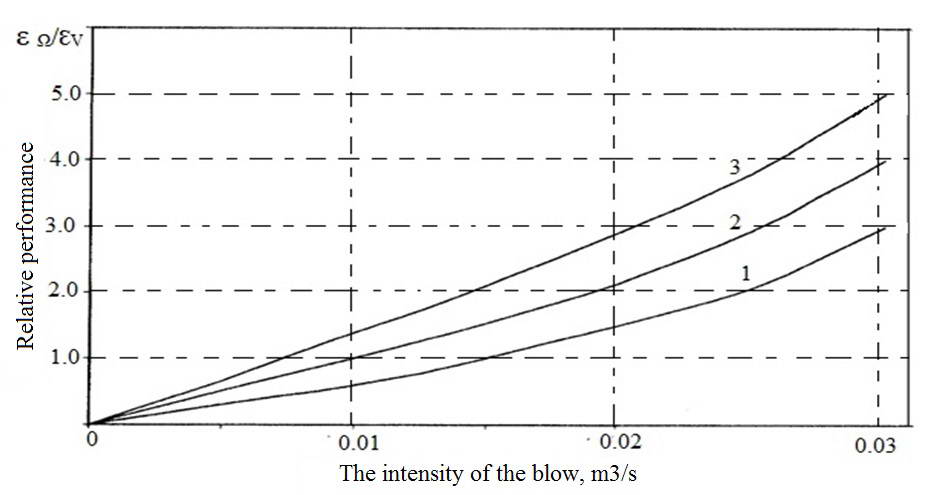
Fig. 5. The dependence of the relative efficiency of the furnace-ladle degassing of the melt copper on the intensity of the blow different depths vacuum
1 - 200 PA; 2 - 400 PA; 3 - 600 PA
Conclusion
In production of ordinary gauge copper, you can refuse from producing vacuum processing of dispersed melt argon blowing with optimal intensity through refractory block with a directional porosity, especially in the period of production of copper from the melting furnace [3].
References
- Фромм Е., Гебхардт. Газы и углерод в металлах. – М.: Металлургия. – 1980. – 712с.
- Линчевский Б.В. Термодинамика и кинетика взаимодействия газов с жидкими металлами – М. – 1986. – 224с.
- Захаров Н.И., Дюдкин Д.А., Туяхов А.И. Моделирование теплофизических процессов внепечной дегазации металлов продувкой инертным газом. – Донецк: Юго-Восток. – 1999. – 140с.
- Левич В.Г. Физико-химическая гидродинамика. – М.: Физматизд, 1959. – 537с.
- Борнацкий И.И. Внепечное рафинирование металлов / И.И. Борнацкий, В.И. Мачилин, В.С. Живченко. – К.: Техника, 1979. – 167с.
- Белов И.В. Влияние массообмена в системе газовых пузырей в жидкости // Журнал прикладной механики и технической физики. – 1989. - №1. – 116-121с.
- Брандт Б.Б. Режим обтекания жидкостью газовых пузырьков // Инженерно-физический журнал. – 1986. - №2. – 197-200с.
- Поздеев Н.П. Влияние расхода аргона на дегазацию расплава в вакууме // Известия вузов. Цветная металлургия. – 1985. - №12. – 59-64с.
- Захаров Н.И. Интенсификация массообменных процессов внепечной дегазации металла // Процессы литья. – 2010. - №4. – 8-12с.
- Захаров Н.И. Массообменные процессы внепечной дегазации металла / Н.И. Захаров, Д.А. Дюдкин, Ф.В. Недолекин, А.И. Туяхов. – Донецк: NORD PRESS, 2009. – 156с.
