Abstract
The substance of the review
- Introduction
- 1. Work purpose
- 2. Research problem definition
- 3. Scientific novelty
- 4. The results received, at the time of writing
- References
Introduction
In the course of burning it is necessary to support an exact ratio between amounts of arriving air and fuel – according to the stekhiometrichesky equation of reaction of burning. When aging equipment mixing is carried out insufficiently precisely, calorific ability of fuel, speed of process of burning and external conditions changes over time. Any of these parameters influences the amount of air necessary for safe and effective combustion of fuel.
Too large amount of air will bring to недожогу and not burned down fuel jumps out a flue, reducing profitability of process and increasing potential danger of explosion. At insufficient quantity the considerable part of heat goes to a flue. Besides, at incomplete combustion of fuel atmosphere pollution increases. At considerable surplus of air the maintenance of SO2 and NOx increases.
1. Work purpose
Development of the measuring device of control of concentration of oxygen in combustion gases of coppers that increases profitability of process of burning and reduces atmosphere pollution by burning products
2. Research problem definition
For achievement of the purpose set in work the following tasks are formulated and solved
- To carry out the analysis and research of methods and technological gages of concentration of oxygen in combustion gases of coppers.
- The analysis of the sizes influencing reliability of determination of concentration of oxygen in combustion gases.
- Development of mathematical model of the channel of measurement on the basis of an electrochemical method with use of firm electrolytes.
- Development of the block of control and regulation of temperature of the measuring instrument of concentration of gases.
- Development of the air filter of the measuring instrument of concentration of gases
- To develop structure of the measuring device of concentration of oxygen in combustion gases.
- To investigate metrological characteristics of the channel of measurement of concentration of oxygen.
- To prove the scheme of the measuring device and to develop requirements to a model sample.
3. Scientific novelty
The mathematical model of the measuring channel on the basis of an electrochemical method with use of a solid-electrolyte cell is developed, the block of control and temperature adjustment that will allow with a sufficient accuracy and speed is developed to define the content of oxygen in combustion gases of coppers
Object of research: The measuring device of control of concentration of oxygen in combustion gases of coppers
Object of research:Increase of profitability of process of burning and decrease in environmental pollution due to use of the developed measuring device in a mode of continuous control of concentration of oxygen in combustion gases of coppers.
4. The results received, at the time of writing
From the carried-out analysis follows that the most suitable gages for control of concentration of oxygen in combustion gases of coppers measuring devices on the basis of firm electrolytes which in the certain range of temperatures possess almost ionic conductivity are. As a sensitive element the test tube from zirconium dioxide which is alloyed by oxide of yttrium or calcium dioxide is used. The characteristic of transformation of this measuring instrument can be described by Nernst's equation [1]:

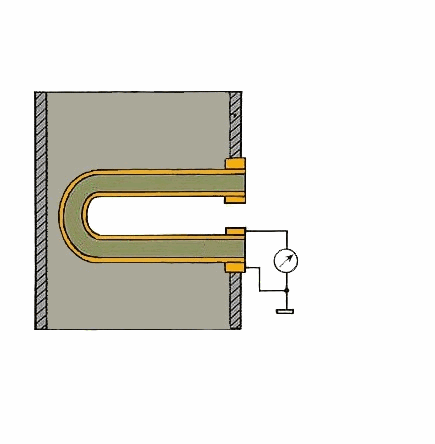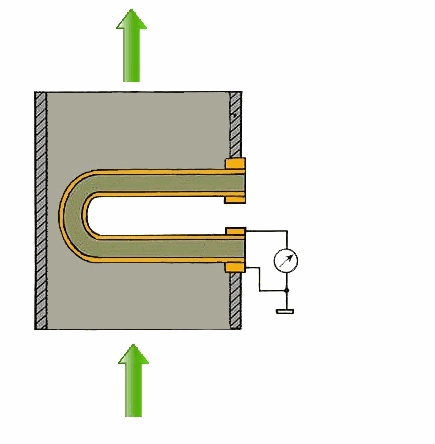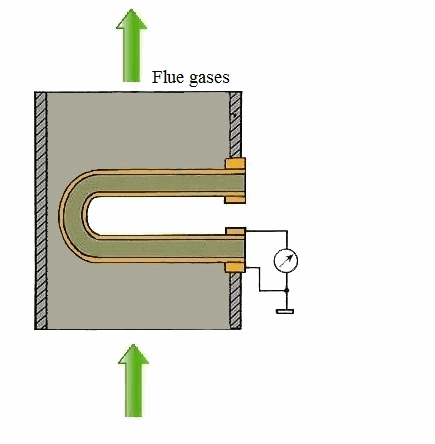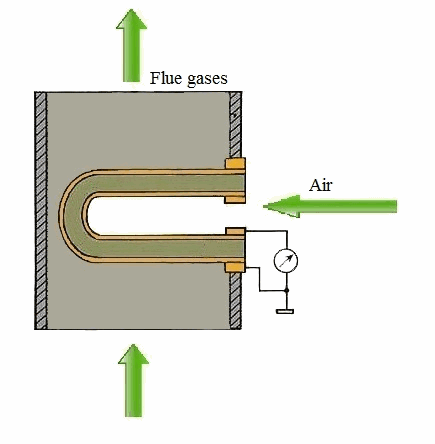
Figure 1 – Principle of action of a solid-electrolyte cell (animation: 5 frames, 5 cycles of repetition, 106 kilobytes)
Development of the block of adjustment of temperature
As a gage of temperature of a tverdoelektorlitichesky cell it is decided to use the platinum thermoresistor which has been switched on in one of shoulders of the measuring bridge
For the solution of a problem of measurement of temperature it is recommended to carry out balancing of the measuring bridge at T = 0oC. We choose the current proceeding through a shoulder of the measuring bridge about. (5 … 10) мА. The supply voltage of the bridge is accepted equal 1.5 V. Tension at the exit of the measuring bridge is described by expression [4] (2):
For reduction of favorable tension of the measuring bridge to the unified look (0 … 5) V for convenience of its further processing the normalizing converter is connected to its exit. (fig 2)

Figure 2 – Voltage at the exit of the measuring converter
The output signal of the normalizing converter bearing in information on change of temperature arrives on astatic on-off the regulator, with a zone of ambiguity presented by expression [5] (3).
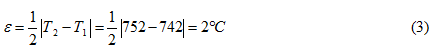
The established operating mode of the block of control and temperature regulation, the corresponding fig. 3, it is accepted to call quasistationary or a mode of self-oscillations. Distinctive feature of this mode is existence of steady harmonic oscillations of temperatures of each of block elements round stationary level corresponding to them. This mode will allow to carry out smooth regulation of temperature of a tverdoelektrolitichesky cell [5]:

Figure 3 – Block diagram of system of thermal regulation
Information about changes in temperature of the object through the thermistor temperature control OT included in the measuring scheme MS arrives at normalizing converter NC. Normalizing converter NC amplifies the signal bridge to the level required for the temperature controller TC. Depending on the received signal, temperature controller TC modifies power supply from the power source PS for executing device (heater) ED.
The schedule of dependence of output tension of TEYa from concentration of oxygen in the range from 1 to 14 about. by % with an excessive pressure of gas mix from –3,9 to 4,4 kPas it is provided on figure 5.

Figure 4 – Dependences of output tension of a solid-electrolyte cell on concentration of oxygen in the range from 1 to 1,4 об.% with an excessive pressure of mix from –3,9 to 4,4 kPas
From the analysis of the given dependence (see fig. 3) follows that
– the maximum level of output tension of solid-electrolyte answers the minimum concentration of oxygen and the minimum excessive pressure of gas mix:

– the minimum level of output tension solid-electrolyte cell of answers maximum:

Sensitivity of solid-electrolyte cell at change:
– concentration of oxygen makes:

– excessive pressure

For increase of sensitivity of the measuring converter of concentration oxygen, and also carrying out scaling of its output signal to the unified level it is necessary to use the analog block of amplifiers. The output signal of solid-electrolyte cell is a differential differential signal of tension. For its strengthening and transformation to a format of entrance signals of the analog-digital converter (ADC) it is necessary to use the analog block for transition from ungrounded to grounded the loadings which block diagram is given in figure 6.
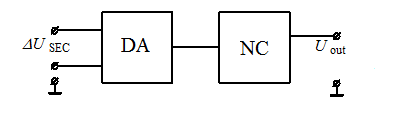
Figure 5 – Block diagram of the analog block of the measuring device of concentration of oxygen
In figure 5 it is designated: DA – the differential (differential) amplifier; NC – the normalizing converter; ΔUSEC – output differential signal of solid electrolyte cell; Uout – output signal of the normalizing converter;
The differential amplifier carries out subtraction of an output signal of TEYa and its preliminary strengthening. For reduction of temperature drift of the amplifier and increase in coefficient of suppression of an inphase component in an entrance signal it is recommended to choose transfer coefficient on DP tension no more (10…15). The normalizing converter carries out function of scaling of an output signal of the measuring channel to a format of an entrance signal of ATsP. Also NP eliminates additive and multiplicative making errors in the analog measuring channel. Range of an output signal of NP makes from 0 to +5.
5. Conclusions
1. The developed mathematical model of the measuring converter of concentration of oxygen on the basis of a tverdoelektrolitichesky cell which considers change of a complex of destabilizing factors: atmospheric pressure, excessive pressure; temperatures of combustion gases.
2. For control of concentration of oxygen in combustion gases with a relative error it is no more necessary to carry out measurement of excessive pressure in the range from –3,9 to 4,4 kPas with a relative error no more.
3. Requirements to temperature installation by the smooth regulator are developed:
– nominal rate of temperature of a tverdoyelektrolitichesky cell oС 750;
– range of change of installation of temperature,oС 750 from 748 to 752;
– value of an absolute error of installation of temperature, oC ±0,3;
– constant of time of a temperature regulator, from no more than 10;
4. At mathematical modeling of a gage are carried out estimates of indicators of sensitivity on the output tension of the measuring device of concentration of oxygen in combustion gases:
– at change of concentration of oxygen 5,33%;
– at change of excessive pressure of 20,6 mV/kPa.
5. Calculation of the channel of measurement of temperature which provides information on change of temperature of a tverdoelektrolitichesky cell is made;
6. As the regulator of temperature the astatic on-off regulator which will provide necessary smoothness of change of temperature was chosen;
7. The block diagram consisting of the channel of measurement of temperature of a tvyordoelektrolitichesky cell, the regulator of temperature giving power from the power supply on an executive heating element is submitted. This block diagram will allow to provide temperature of a cell at the level of 750 °C, with an accuracy of installation of temperature ±2 °C aren't worse.
In writing this essay master's work is not yet complete. Final completion: December 2014.
References
1. Аналитическая химия: учебник: в 2-х кн., кн. 2: Физико-химические методы анализа / Под. ред. В.П. Васильева. – [5-е изд., стер.]. – М.: Дрофа, 2005. – 383 с.
2. Справочник химика: в 6 т. Т. 4: Аналитический анализ, спектральный анализ, показатели преломления /Под. общ. ред. Б.П. Никольского. – Л.: Химия Ленингр. отделение. – 1967. – 920 с.
3. Гофман М.А. Повышение точности измерения концентрации кислорода в цифровых твердоэлектролитных газоанализаторах / М.А. Гофман, М.В. Колечкин, О.И. Потатуркин, П.А. Чубаков // Автометрия. Российская академия наук. Сибирское отделение. – 2000. – № 6.– С. 82 – 87.
4. Геращенко О.А., Гордов А.Н., Лах В.И., Стаднык Б.И., Ярышев Н.А. Справочник: Температурные измерения – К.: Наукова думка, 1984 – 495 с.
5. Грабой Л.П., Ленская Л.П., Трощенко А.В. “Определение динамической ошибки регулирования двухпозиционного термостата”, Вопросы радиоэлектроники, ТРТО, вып. 1, 1971.
6. Дьяков В.И. Типовые расчеты по электрооборудованию, М., Высш. шк., 1991 – 158 с.
7. Вечер А.А. Твердые электролиты. / А.А. Вечер, Д.В. Вечер Д.В. Минск: Университетское изд., 1988. – 110 с.
8. Вовна А.В., Левшов М.М. Разработка математической модели измерительного прибора концентрации кислорода в дымовых газах. Автоматизация технологических объектов и процессов. Поиск молодых. 2013г. г. Донецк.
9. Вовна А.В., Левшов М.М. Разработка блока контроля и регулирования температуры измерителя концентрации газов. Автоматизация технологических объектов и процессов. Поиск молодых. 2014г. г. Донецк
10. Вимірювання температури: теорія та практика / Луцик Я.Т., Гук О.П., Лах О.І., Стадник Б.І. – Л. : Бескид Біт, 2006. – 559 с. – ISBN 966-8450-25-6
