Content
- Introduction
- 1 Dephosphorization of steel
- 1.1 Dephosphorization of steel under reducing conditions
- 1.2 Dephosphorization of steel in vacuum
- 1.3 Dephosphorization of steel in high-alloy steels
- List of sources used
- STUDYPORT [Электронный ресурс], 2020 – Режим доступа: https://studyport.ru/referaty/tehnika...
- VT-METALL металлообрабатывающая компания [Электронный ресурс], 2020 – Режим доступа: https://vt-metall.ru/articles...
- Т.3: Сборник научных трудов Черная металлургия России и стран СНГ в XXI веке / Москва, 1994.
- Краснянская, И.А. Исследование закономерностей удаления фосфора и се-ры из оксидных расплавов для разработки технологии предварительного ра-финирования сплавов железа в печах барботажного типа: дис. на соиск. учен. степ. канд. техн. наук (05.16.02) / И.А. Краснянская. – Москва, 2015. – 127 с.
- Т.2: Сборник научных трудов Черная металлургия России и стран СНГ в XXI веке / Москва, 1994.
- Способ дефосфорации легированного металла в электропечи // Патент России № 2009208. 1994. Родинков С.В., Антипов В.М., Гутовский И.Б., [и др.].
Introduction
Phosphorus enters steel mainly with the original iron used for steelmaking.
Phosphorus has a high tendency to segregation, therefore, even with an insignificant average amount of phosphorus in the casting, areas rich in phosphorus can always form.
Phosphorus located near the boundaries increases the temperature of transition to the brittle state (cold brittleness). Therefore, phosphorus, like sulfur, is a harmful impurity, its content in carbon steel is allowed up to 0.050% [1].
Segregation of phosphorus (P) to a much lesser extent than sulfur and carbon occurs during the solidification of steels. The higher the percentage of phosphorus the steel contains, the higher its cold brittleness and the lower the impact strength and plasticity.
The high temperature of the medium makes it possible to achieve the solubility of phosphorus within 1.2%. The lower the temperature becomes, the lower the phosphorus solubility. It gradually drops to 0.02–0.03%. This may indicate that, as a rule, it is completely dissolved in the alpha gland.
The temper brittleness of chromium, chromium-nickel and chromium-manganese, manganese and magnesium-silicon alloy steels largely depends on the segregation of phosphorus along the grain boundaries. The element helps to slow down the decomposition of martensite and increases the hardenability.
The presence of phosphorus in chromium-nickel austenic steels leads to an increase in the yield strength. When austenic stainless steel is exposed to a strong oxidizing agent, the presence of phosphorus in its composition causes corrosion at the grain boundaries. This behavior is predetermined by the segregation of phosphorus at these boundaries. [2].
Next, we will consider the principles of phosphorus removal under various conditions.
1 Dephosphorization of steel
1.1 Dephosphorization of steel under reducing conditions
This dephosphorization technology is promising for steels containing significant amounts of chromium, manganese, carbon, or other active elements.
According to the classical technology, the removal of phosphorus from liquid steel takes place during the period designated conditionally from the moment of the end of the melting of the charge to the oxygen purging of the bath. Along with phosphorus, at the same time, a significant part of the expensive alloying materials contained in the steel is oxidized, which are irretrievably lost with the slag removed from the furnace.
According to the new technology, it is proposed to refine metal from phosphorus during the recovery period of smelting. In this case, the dephosphorization of steel is carried out after the operation of deoxidation and desulfurization of the metal, or is combined with these processes. As a result, there is practically no waste of elements, and the refining slag contains minimal amounts of valuable alloying oxides.
Metals of alkaline and alkaline-earth groups or their compounds are used as dephosphorizers. So, when using calcium, the process of removing phosphorus from liquid iron is described by the reaction 3(Са) + 2[Р] = (Са2Р2), and the change in the standard free energy AF° = 88230 + 40,4Г, cal.
The complete development of the refining process is facilitated by lowering the bath temperature. Calculations have shown that a decrease in the temperature of the metal melt by 100 °C reduces the equilibrium concentration of phosphorus in steel by more than 2 times. Thus, the temperature factor is important for the successful implementation of reductive dephosphorization of steel.
It is also known that calcium has a significantly higher affinity for oxygen than for phosphorus. Therefore, for the predominant combination of calcium with phosphorus, non-oxidizing conditions are required. In practice, they can be provided due to the deep deoxidation of the metal bath, the low content of iron monoxide in the slag phase and the minimum oxygen content in the furnace atmosphere.
The above reaction is reversible. Therefore, when the specified conditions change, metal re-phosphorus is possible. Hence, the duration of the treatment of the bath with the reagent-dephosphorizing agent becomes important. The dephosphorization efficiency can also be influenced by the way calcium is introduced into the furnace.
After determining the necessary technological parameters, the process of reducing dephosphorization was tested in 0.3 tons of a DC plasma arc furnace at the experimental base of TsNIIchermet and in a 3 tons arc furnace at one of the machine-building plants. The plasma-forming gas was commercially pure argon. Metallic calcium or calcium carbide (the latter mainly for carbon steels) was used as a dephosphorizer. The charge consisted of 100% steel waste of a given grade. After complete melting of the charge, the slag was downloaded (in whole or in part), two types of deoxidizers were added to the metal, then refining slag was added from a mixture of lime and fluorspar, as well as a powdered deoxidizer in a slightly altered proportion as compared to the conventional technology. The oven was sealed. Then, a dephosphorizer was introduced into the furnace in portions for ~ 15 min. After the end of the reaction, the phosphorus-containing slag was completely removed from the furnace. Further operations were carried out according to well-known technology.
The degree of phosphorus removal from liquid steel using the new technology (during the recovery period of melting) reached 40%. At the same time, the content of alloying elements remained unchanged. Simultaneously with phosphorus, up to 50% of the sulfur contained in the steel was removed.
Experiments in a 3t arc furnace confirmed the effectiveness of the new technology of steel dephosphorization [3].
1.2 Dephosphorization of steel in vacuum
The degree of phosphorus removal is markedly reduced in the presence of iron oxides in the melt. The ability to remove phosphorus oxide from a compound with iron by reaction
12(FePO4)=4(Fe3O4)+6{P2O5}+{O2}
can be estimated using the data of thermodynamic modeling of the program HSC Chemistry 6.0 (picture 1)

Picture 1 - Results of thermodynamic modeling of the behavior of iron phosphate depending on temperature
The graph shows that at Т=1500 °С there are no strong compounds of phosphorus and iron, and phosphorus must be in a gaseous state. Let us estimate the temperature at which in such a system the pressure P2O2(г)=1 atm and taking into account the activities of the components in the melt, phosphorus passes into the gas phase.
The temperature dependence of the reaction equilibrium constant can be determined using data from the HSC Chemistry 6.0 software (Table 1).
Table 1 - Dependence of the reaction equilibrium constant on temperature

The temperature dependence of the equilibrium constant is expressed by the equation:
![]()
The dependence of the possibility of the transition of phosphorus to the gas phase on temperature was obtained. It was taken into account that Pp2o5=1 atm. However, if the process is carried out in a vacuum, then a more complete course of the process of removing phosphorus into gas should be expected. Purging with inert or oxidizing gases can have a similar effect.
1.3 Dephosphorization of steel in high-alloy steels
A number of directions can be identified in solving the problem of removing phosphorus from high-alloyed melts based on diluting the charge with low-phosphorous materials, dephosphorization by the electrochemical method, treatment with slag mixtures under reducing conditions, dephosphorization by the formation of volatile phosphorus compounds (halogens, phosphine, etc.), binding phosphorus by phosphide-forming elements (В, Nb, Zr, V, Са, Mg, RZM).
Analysis of the economic, technological and environmental aspects of the above methods allows us to conclude that the most promising areas for the refining of high-alloy steels and ferroalloys from phosphorus should be considered slag dephosphorization under weakly oxidizing conditions, the transfer of this element into the gas phase and its binding into stable non-metallic inclusions such as phosphides.
For melts, where the main alloying components are W, Cr and, to a certain extent, V, it is advisable to carry out dephosphorization under low oxidizing conditions (10 ~ 14 atm.), Which makes it possible to significantly simplify the removal of phosphorus and avoid noticeable loss of alloying. However, in this case, the use of traditional furnace slags is ineffective. For example, for steel of type Kh18N10, the partial pressure of oxygen should not exceed 10-12 atm. (this corresponds to the equilibrium of a metal melt containing 18% P, with a slag saturated Сг203 at 1823 К). At such values of p, oxidative dephosphorization can be effectively carried out only with the use of fluxes with a high phosphate capacity C. Such refining mixtures include systems СаО-CaF2; BaO-BaCl2: BaO-BaF2; NaO-SiО2; BaO-MnO, the sorption capacity of which in relation to phosphorus is 46 orders of magnitude higher than the capabilities of furnace slags.
At the same time, it is necessary to note the instability of the results obtained for the removal of phosphorus from high-chromium melts, which is primarily due to the kinetic difficulties of the process due to heterogenization of the slag and a decrease in its phosphate capacity due to the transfer of chromium from the metal into it.
Dephosphorization due to the transfer of phosphorus to the gas phase. On the basis of thermodynamic analysis of the process of dephosphorization of corrosion-resistant steel of the Kh18N10 type during treatment with gas mixtures containing water vapor and slag with a low oxidation potential, the fundamental possibility of transferring phosphorus from a highly alloyed melt into the gas phase is shown. Two mechanisms of phosphorus removal are considered: according to the first, phosphorus in pure form or in the form of compounds passes into the gas phase; on the second, phosphorus first passes into slag, and then into the gas phase [4].
The dephosphorization process can be facilitated if carbon is artificially introduced into the reaction zone, for example, in the form of graphite.
The closest in technical essence and the achieved result is the method of dephosphorization of steel in a high-frequency induction furnace. Magnesite crucible. The furnace is additionally equipped with a cover to isolate the melting metal from the air atmosphere [5].
According to the prototype method, after complete melting and heating of the metal to the required temperature, the metal is deoxidized with aluminum by 0.3-0.4%, the furnace is covered with a lid, the melting space is filled with argon at a flow rate of 2 nm3/h and a dephosphorizing mixture consisting of Ca-CaF2, or calcium carbide in the amount of 20-25 kg / t and fluorspar in the amount of 6-10 kg / t. After dephosphorization, the duration of which is 10-20 minutes, the phosphorus-containing slag is discharged from the furnace and subjected to oxidation in order to prevent harmful emissions PH3 into the atmosphere of the workshop. As a result, the phosphorus content in high-chromium steel decreased from 0.030% to 0.016%, i.e. by 47% [6].
Calculation of reactions of emission of harmful gases PH2 as: P + 2H(g) = PH2(g) и PH3 as: Ca3P2 + 3H2O = 3CaO + 2PH3(g) in the HSC Chemistry 5 program showed the following results (tables 2, 3):

Table 2 - The results of calculating the reactions of the release of harmful gases in the HSC Chemistry 5 program

Table 3 - Characteristics of substances involved in the reactions
Dependence ΔG temperature for two reactions is shown in Pictures 1 and 2.

Picture 1 – Dependence ΔG on reaction temperature P + 2H(g) = PH2
Picture 2 – Dependence ΔG on reaction temperature Ca3P2 + 3H2O = 3CaO + 2PH3(g). Reactions proceed towards obtaining reaction products. The characteristics of the substances involved in these reactions are given in Table 4. Table 4 – Characteristics of the substances involved in the reactions When writing this abstract, the master's work has not yet been completed. Final completion: November 2021. Full text of the work and materials on the topic can be obtained from the author or his manager after that date.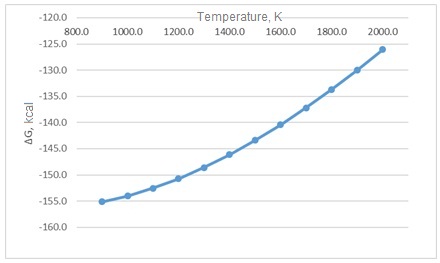

List of sources used
