|
ARTICLE
|
Metallurgy of High Chromium-Molybdenum White Iron and Steel Rolls
INTRODUCTION
The Fe-Cr-C system is the basis of a number of the most widely used wear resistant materials having application in mining, mineral processing, cement works, agricultural sectors and rolling mill rolls.
The iron-chromium aliens were studied as early as 1892 by F. Osmond who was the first to indicate the presence of complex carbides. Other investigations have been carried out by other authors such as W. Tot ante and K. Bungardt.
In the frame of research works sponsored by the Climax Molybdenum, a very important development in the know ledge of the metallurgical properties of those alloys was done by F. Maratray.
As concern rolling mill rolls in reviewing the history of the manufacture of rolling mill rolls, the most important innovation was probably the introduction of the double-poured indefinite chill roll in the late thirties. However, European experience may suggest that the introduction of the high chromium iron and steel rolls is likely to rank as another significant innovation in the art of rollmaking.
Some Russian and American papers relate the use of high chromium iron for the manufacture of small bars and heavy section mills rolls in the early sixties. The first attempt to use that iron for hot strip mill rolls was made in Germany in 1965. Other experimental rolls were made in England a little later. It became quickly clear that despite of some failure due to the lack of reliable metallurgical information of the high chromium iron system, those rolls had an excellent resistance to both abrasion and banding. They become quick the standard grade used in the earl finishing stands of Hot Strip Mills.
Some years later, another improvement was done with the development of the high chrome steel. Carbon and chromium content were strongly decreased. Special heat treatment or alloying element additions gives a material, which suits very well to the working conditions in roughing stands of Mot Strip Mills. Today, high chrome steel rolls are used world-wide.
PRODUCTION TECHNIQUE
The first rolls have been produced by the double-pour method. The practice was very similar to that used for double-pour indefinite chill rolls, except that a much greater volume of flush iron must be used to reduce the chromium content of the roll core to an acceptable level. Some trials have also been done to produce those rolls single-pour and by electroslag melting of a sleeve remelted around a premachined steel arbour.
Actually, all the high chromium iron and steel rolls are manufactured by centrifugal casting technique. The process can be vertical, horizontal or even tilted. By this technique, the small volume of the high chromium shell can solidify more rapidly than with the other casting methods thus obtaining finely dispersed carbides. It can be told that the development of centrifugal casting as a new process for rollmaking has been strongly connected to the implementation of high chromium rolls.
Core is made of lamellar or spheroidal graphite (S.G.) iron. The introduction of new rolling technology such as heavy bending, shifting and crossing increases strongly the mechanical stresses in the journals and core. For that reason most of the high chromium rolls are now cast with a S.G. graphite iron core which has higher mechanical properties than lamellar iron ones.
METALLURGY OF HIGH CHROMIUM IRON AND STEEL ROLLS
In Fe-Cr-C alloys, chromium can substitute to iron in cementite up to a 15% content. For higher content. cementite become unstable and is replaced by an hexagonal carbide whose composition is M7C3. Those carbides called chromium carbide contain mainly chromium and iron, but other alloying element can be present.
It is generally accepted that the most significant reason for the good abrasion resistance of those materials is the presence of the chromium carbides in the microstructure. Hardness of the chromium carbide is in the range 1500-1800 Vickers compared to the cementite whose hardness is in the 1000-1200 range.
Most of the studies of the Fe-Cr-C system are based on the diagram published by Jackson and given on figure 1. The y-M7C3 area is crossed by a eutectic which is limited by two peritectic lines. The y-8 for low carbon and high chromium content and M7C3-M3C for high carbon and low chromium content. The position of the late depends strongly of the cooling speed. High cooling speeds promote the M3C formation.
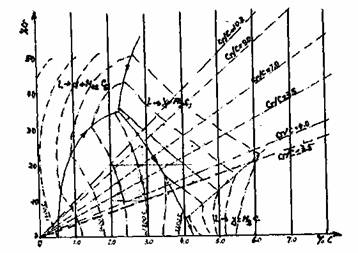
Fig. I : Liquidus surface of Fe-Cr-C diagram
The eutectic structure depends on the amount of austenite formed at the start of solidification. When the austenite leaves only a small volume after solidification, carbide have a tendency to form along the rain boundaries as shown in figure 2.a. This is the carbide morphology of a chrome steel rolls. With carbide content of 20 to 30%, the eutectic contains lamellae radiating from the centres located in the interdendritic spaces (figure 2.b). This is the general carbide structure of a chrome iron roll as used worldwide in the early finishing stand of Hot Strip Mill. This structure changes to a lamellar one when the austenite no longer interferes with the eutectic formation. Finally, with 35 to 40% of carbides, the alloy becomes hypereutectic and large hexagonal primary carbides appear. From our knowledge, no rolls are produced with such a low toughness structure.

Fig. 2a : Typical microstructure of high Cr steel roll

Fig. 2b : Typical microstructure of high Cr iron roll
A low casting temperature and a rapid solidification will decrease the size of the eutectic cells. No significant change in the morphology of the eutectic carbide occurs in during heat treatment operations, which are always performed on the roll after casting.
The percentage of chromium carbides can be calculated approximately from the C and Cr content of the alloy by the following formula:
% carbides = 12.33 (%C) + 0.55 (%Cr) - 15.2
The chromium content of the matrix increases regularly with the chromium/carbon ratio and can be represented by the equation :
% Cr matrix = 1.95 (%Cr/%C) - 2.47
The following table gives the typical range of carbon and chromium content as well as the percentage of carbide and chromium content of the matrix measured on the high chromium grades that are used actual in hot rolling.
|
|
%C |
%Cr |
% carbides |
Cr/C |
% Cr matrix |
|
Hi-Cr steel |
1.0/1.5 |
11.0/12.0 |
5/15 |
8/10 |
10/14 |
|
Hi-Cr iron |
2.5/3.0 |
16.0/18.0 |
25/30 |
5/7 |
6/10 |
The large difference in matrix composition for the two alloy families induces two distinct oxidation behaviours in the Hot Strip Mills. High chromium irons with their low Cr content in the matrix will oxidise three times faster than the high chromium steels.
As concern the metallic matrix, all the decomposition product of the austenite can be produced: pearlite. bainite, martensite. A full austenitic matrix can also be completed and in many structures retained austenite is present.
However, for most of the applications as rolling mill rolls of the high chromium alloys, a microstructure in which the matrix has a low residual austenite level and is free of pearlite is required. In order to get such a structure on pieces weighting more than 7 tons and which may exceed 40 tons, different process conditions have to be put under control.
Those are :
• Tempering heat treatment
• High temperature heat treatment
• Alloying elements addition
Depending of their own facilities, most of the rollmakers use one or more of the above mentioned method in order to get the required structure and hardness. Those manufacturing conditions interfere between them.
TEMPERING HEAT TREATMENT
During a slow cooling, in the high temperature range, austenite is saturated in carbon and a precipitation of small secondary carbide may be observed (figure 3). Such a precipitation decreases the carbon and chromium content of the matrix. The pearlitic transformation is delayed and a bainito-martensitic transformation becomes possible at low temperature. Figure 4a and 4b shows the effect on the destabilisation of the austenite on the isothermal transformation diagram and on the martensitic transformation.
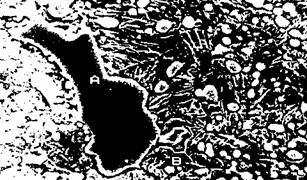
Fig. 3 : Typical secondary carbides precipitation
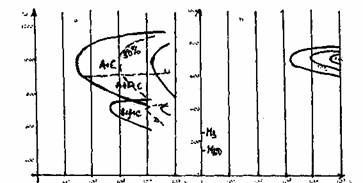
Fig. 4a: Schematic isothermal transformation diagrams.
a) undestabilized austenite
b) austenite destabilized by precipitation of secondary carbides
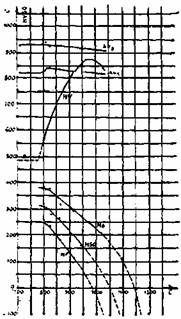
Fig. 4b: Effect of destabilization temperature on transformation temperatures and the Vickers hardness as air-cooled after destabilizing of a high Cr iron.
It is believed that these secondary carbides, apart from their effect on the transformation of austenite to martensite contribute to wear resistance because of their high hardness and because of their dispersion hardening effect on the matrix.
In the as-cast condition, the matrix contains a high percentage of austenite (30-60%) which has to be destabilised by one or several tempering heat treatment in order to get the specified hardness with a structure containing M^Q, carbides in an α matrix.
However there is always a stable austenite residue. If the temperature of the tempering treatment is raised in order to decompose it. hardness will be decreased. There is therefore a limit to the efficiency of those low-temperature heat treatments. If a hardness of more than 80 shore C is required, it cannot be reached with a minimum of residual austenite.
Figure 5 shows for a 17% Cr - 2.8% C HSM work roll the hardness and residual austenite content evolution versus the Larson - Miller parameter P which equals:
P= (273+T)(20+log t) where T is the temperature (°C) and t the soaking time (hours) of the tempering heat treatment.
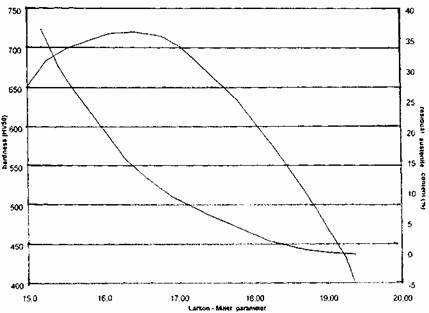
Fig. 5: Influence of tempering temperature on hardness and residual austenite content
HIGH TEMPERATURE HEAT TREATMENT
If this procedure is standardized, slow cooling rate can be used after initial solidification of the roll. Rolls produced in this way may have in the as-cast condition a hardness as low as 50 Shore with a pearlitic structure and can be premachined in such condition. After that, the specified hardness can be obtained by reaustenizing the roll at high temperature followed by a controlled cooling up to room temperature. The controlled cooling allows a good control of the precipitation of secondary carbides in the temperature range of 800 to I050°c. Using that procedure, the roll metallurgist can produce and control the microstructure. After that quenching or controlled cooling, the roll is tempered to the hardness required.
High temperature heat treatments produce a more uniform matrix homogeneity and structure than that which can be produced by controlled cooling alone. This is due to the fact that the macrosegregation phenomena cannot be neglected in those alloys. In case of rather fast solidification as in a spun-cast roll, we observe a higher chromium content in the core of the austenite cells than close to the eutectic carbides as shown on figure 6. It is clear that the diffusion connected to a high temperature heat treatment in the 1000/1050 °C range allows to homogenize the gradient of the elements in the austenite cells.
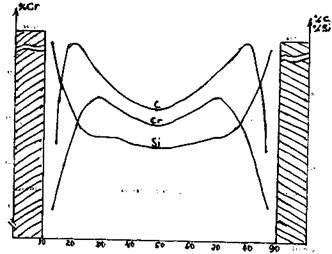
Fig. 6: Distribution of elements (Cr, C, Si) across a dendrite
Such heat treatment operations are rather expensive due to the slow heating rates that are required for massive metal sections such as rolls. They give however the best control of the hardness and of the component of the microstructure.

