Picture 1 - Classification of nonmetallic inclusions.

Faculty: Physical Metallurgy
Speciality: Ferrous metals
Along with the homogeneity of composition and the minimum content of harmful impurities primary qualitative characteristic of steel is its degree of purity of non-metallic inclusions, which affects the anisotropy of properties, cold brittleness threshold, weldability, the propensity to aging, technological plasticity, contact strength, etc.
The urgency of the problem of "clean steel" is due to the continuous tightening of requirements for the consumers to the quality of the metal. According to modern concepts, defining influence not only the absolute content of inclusions in the metal, but their composition, shape, distribution and deformation during rolling.
Repeatedly observed significant differences in some of the mechanical properties, despite the same chemical composition and structure of the metal. Based on the fact that the concept of clean steel is relative, because for every ton of steel accounts for 1012 - 1015 oxides, and the amount of sulphides and oxysulphides - the value of the same or greater order, we should accept the inclusion of natural structural components. The problem of quality therefore largely be addressed optimizing the composition and structural components of steel - the inclusion, ie,. most high performance steel. Study of the nature and properties of the inclusions from this perspective fell far short of studies of metallic phases.
Previously, the properties of steel products, in particular, mechanical associated with concentrations of oxygen and sulfur: new studies have shown the decisive influence in the kind, size and distribution of steel oxides and sulfides. Have made great strides in steel production of high purity, however, is no doubt that industrial steel utility will contain a significant amount of inclusions.
Consequently, instead of taking costly measures to reduce the total content of inclusions to the increasingly low levels, would bring great benefit to the efforts aimed at regulating the composition and morphology of inclusions. This would allow the use of the positive characteristics of the inclusions and to guarantee the conditions under which the negative effects of inclusions would not have led to potentially dangerous situations.
Two decades have established the concept of "clean steel" or the degree of purity, depending on the contamination of steel by nonmetallic inclusions of different composition and morphology. Therefore, some puzzling assertions AP Gulyaev, according to which, contrary to conventional ideas purity became associated with the presence only of harmful impurities, and, according to the classification of impurities NT Gudtsova, these include sulfur, phosphorus, gas, nonferrous metals . Excluding the effect of nonmetallic inclusions, the author believes that "the difference in the properties are different modes of production due mainly to the content" harmful substances "that contradicts current concepts about the role of secondary phases, in particular non-metallic inclusions.
Formation NI possible in the presence of the substrate, ie when in solution are already present centers of origin of a new phase.
When deoxidation of the metal dominates the heterogeneous nucleation NI, because the melt existing centers of nucleation of new phases such as:
1. Oxides covering the reducing agent (aluminum, ferrous alloys are always covered with oxides as a result of contact with air).
2. Metal is not absolutely clear from the NI, even before entering the reductants. After the oxidation period is not the whole mass of oxidized elements float to the slag.
3. The surface of the lining can also serve as centers of nucleation of a new phase.
From the above, we can conclude that part of the NI formed a heterogeneous way as in the metal enough nucleation centers of a new phase. At the moment I deoxidizer are formed zone in which the concentration of deoxidizers great that promotes homogeneous nucleation NI. From the above, it follows that the formation of NI is not unhindered.
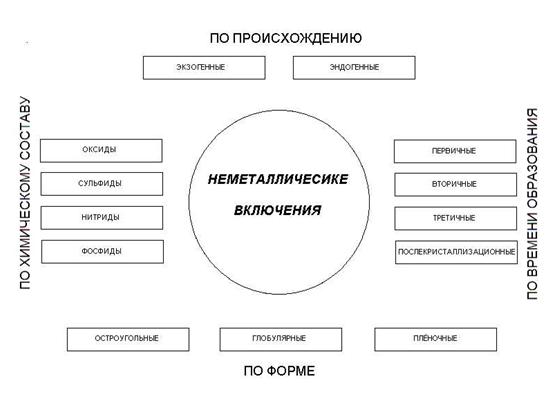
Inclusion can be classified to the following factors:
• By origin
• The chemical composition
• At the time of formation
• The shape of
The foregoing is shown in picture 1.

Picture 1 - Classification of nonmetallic inclusions.
Operational properties are determined primarily by the shape, size and distribution of inclusions, which depend on the method of reduction, the conditions of solidification and deformation. Often it is very important not to achieve a very low absolute contents of NI, and receipt of inclusions with optimal properties and to processes of phase separation and strain and possibly less harmful impact on the workability and performance of the steel.
Many researchers believe [5] that the type of inclusions does not affect the mechanical properties of steel. The determining factors are considered critical size of inclusions, which is set for many materials. Apparently, for each steel there exists a critical size of inclusions, depending on the type of inclusions, steel and placed on her property. Larger inclusions are dangerous to have, regardless of their composition. Microscopic inclusions, whose size is less critical, do not behave as defects.
The least harmful to the properties of steel many brands of globular silicate, fine and uniformly dispersed inclusions. Less favorable crystalline, almost correctly limited the inclusion of: titanium nitride, corundum. It is believed that such inclusion because of its non-plastics in metal deformation are stress concentrators. Even more damaging accumulations (in the form of swarms or clouds), a large number of small inclusions, sometimes leading to the appearance of visible defects (bundles) metal (it is more typical for the metal is poured into molds). Steel containing lamellar inclusions, destroyed much earlier steel with globular inclusions [5]. Very unfavorable influence of chain location of nonmetallic inclusions, when the cast grain boundaries are located in a chain of several low-melting and easily deformable inclusions. Such inclusions weaken the cohesion between individual grains, and sharply reduce the strength and plastic characteristics of steel. Chains make more sulfur and less oxysulfide inclusions [3].
For homogeneous nucleation of nonmetallic phase requires large supersaturation of the initial components. When deoxidation of metal such components are oxygen and reducing agent. Necessary supersaturation of the initial components are reached at the time of dissolution and homogenization introduced into the metal deoxidizers. Therefore, the formation of nonmetallic inclusions is unimpeded.
If you calculate the equilibrium composition of NI averaged chem. composition of the metal, the current composition of the inclusions will not reflect the true picture. For example, there are inclusions, consisting of almost pure Al2O3, although the metal does not contain an equilibrium with aluminum and oxygen. The reason is that after the introduction of deoxidizers in metal-rich zone formed reductants, resulting in NI contain a high percentage of oxide deoxidizers. As averaging deoxidizers in the metal formed NI containing fewer oxide deoxidizers. To take into account the above, the model calculation of the NI proposed the following approach.
After entering deoxidizer metal conventionally divided into three zones:
1. Metal, enriched with oxygen;
2. Metal-rich reductants;
3. The reaction zone (zone of Formation NI).
In the reaction zone receives a certain amount of metal-rich reductants (Omor) and thethe volume of of metal ,oxygen-enriched( Omoks),and calculates the equilibrium composition of the metal and NI ( andamounts )on the average chemical composition of the reaction zone ( averagetotal of Omor and Omoks ) . By stirring in a while the reaction zone is updated with new volumes of metal from the zones 1 and 2. Reacted volumes averaged in zones 1 and 2, and to replace them come new Omor Omoks and then the entire calculation is repeated. Update rate of the reaction zone is calculated by the mixing intensity. Due to the rupture zone 1, the turbulent flow increases its contact area with the Zone 2 is increases the reaction zone, which in turn is also calculated by the mixing intensity.
Calculation of the removal of NI due to assimilation of slag produced in a similar way, only in this calculation, the first zone is a metal, a second slag, the third is the surface of the metal-slag (the zone in which the act of transition NI metal in the slag). How quickly the NI to the surface of the metal-slag and the rate of its average in the slag is calculated from the intensity of mixing. The act of transition metal in the NI of slag in the third zone is estimated by the work of adhesion.
Removal of NI due to assimilation of the slag depends on the wettability of the inclusion of slag. Data or functional dependencies wettability nonmetallic slag on the chemical composition of slag over a wide concentration range practically does not exist. And so in order to evaluate (or compare) the carrying capacity of slag nonmetallic inclusions suggested the following approach.
It is believed that the results of removing the nonmetallic inclusions in the aging steel before casting the better, the more opportunities for the coagulation of low-melting mixtures of oxides. At the same time should be given great importance to the selection of reductants to already react with dissolved oxygen formed in the metal inclusions, which would immediately coagulated into large, easy to remove particles. From this perspective, it is necessary to ensure the fluid mobility NI. This can be achieved at the expense of a certain order additive reductants. To ensure zhidkopodvizhnost NI must fulfill the following conditions:
1. Deoxidation in ascending order of the melting point of the deoxidation products.
When such a sequence of additives reductants formed NI with low melting point will be mainly zhidkopodvizhnye. These zhidkopodvizhnye inclusions can act as nucleation centers for NI additives following portions deoxidizers. This sequence promotes the kinetics of low-melting mixtures of oxides.
2. Deoxidation in ascending order of affinity for oxygen deoxidizers.
Sequence of additive reductants in order of increasing affinity for oxygen also contributes to the formation of low-melting mixtures of oxides. This is due to recovery of components NI reductants.
3. Deoxidation in descending order of the interfacial energy deoxidizers.
With order entry reductants in order of decreasing interfacial energy on the boundary with the liquid metal reducing agent binds most of the oxygen and the products of reduction can be easily removed. After that, the metal remains a small fraction of oxygen and when you enter reductants (alloy) having a low specific interfacial energy of their oxides almost completely dissolved in the metal is not oxidized (ie, well-wetted NI are formed in small quantities). However, when you enter a strong deoxidizer first in large quantities in the metal will dissolve much of the element deoxidizers. And cooling the metal solubility limit, the element deoxidizer reduced due to what form new NI.
4. Deoxidation in descending order of the solubility limit deoxidizer element in steel.
Low oxygen content is achieved through the presence of dissolved element deoxidizers in the steel. Therefore, for deep deoxidation element of the reducing agent should possess high solubility limit in the metal. Elements having a low value pridelnoy solubility in the metal can not be deeply raskislit metal even if they have a great affinity for oxygen. Therefore deoxidants having low solubility pridelnuyu effectively give a last resort. For example, Ca has a high affinity for oxygen and low pridelnuyu solubility. Its usually give a last resort.
The above thermodynamic calculations showed that the removal of nonmetallic inclusions (Al2O3) is necessary to carry out low basic slag. Just because of the presence of highly basic slag surface-active sulfur (at the metal-slag), which prevents the removal of NI, NI recommend removing as low basic slag. As a result, proposed scheme of secondary treatment, where should first direct nizkoosnovny slag to remove the NI and, after the conduct of desulfurization inducing highly basic slag by lime additive into the bucket.
In the process of deoxidation and alloying of steel are formed non-metallic inclusions. The main objective of reduction is the reduction of oxygen, but the process of reduction is accompanied by the formation of nonmetallic inclusions (NI). The chemical composition and physical properties of the NI is determined by the choice of reductants and sequence of their entry into the metal.
Deoxidizers in terms of education NI can be classified as follows:
1. The affinity for oxygen;
2. Formed by the melting point NI;
3. Specific interfacial energy on the boundary of NI - metal;
4. According to the solubility limit of the element deoxidizers in the steel.
Available in a metal bucket strongly pereokislen. In addition, there is a strong enough secondary oxidation of the jet (stream protection is absent). Doping of silicon produced ferrosilicon (FS), silico-manganese doping is (MNT), which also contains silicon. If at first gave the PS and then SMN or SMN and FS were given to both, the oxygen would oxidize the metal in the first silicon, which is "protected" would in this case manganese from oxidation. SMN and FS at a price not much different, and therefore the return of FS first tangible effect in terms of saving ferroalloys will not. In fact, using the sequence of returns is as follows: first, MNT, then ferrosilicon, which allows the formation of more favorable NI. When such a sequence of return of ferroalloys (in order of increasing affinity deoxidizer oxygen) are formed zhidkopodvizhnye NI. What really confirmed by experimental data from the literature.
When using carbon ferromanganese (PSK) should be given small amounts of ferrosilicon. And the number of deoxidizers should be increased. In this case the image NI will have multimodal composition. PSK use is undesirable because to obtain favorable NI must simultaneously give FS. Therefore, the MNT has advantages compared with the PSK from the viewpoint of forming NI. Next to the deep deoxidation can introduce a small amount of aluminum. Before putting the aluminum oxygen content is low (because most of the oxygen linked silico-manganese and ferrosilicon) and therefore the image will include Al2O3 small sizes. Solution of aluminum partially restore the available NI, and eventually became NI will have multimodal composition of the type MnO - SiO2 - Al2O3. And some will include a mixed bag. Al2O3 predominantly wakes is on the outer side of the inclusion, due to which the inclusion of wakes have a low wettability, which contributes to its removal from the metal.
For the favorable composition of NI, the following scheme of reduction:
First introduced SMN, the second PS and the last introduces small amounts of aluminum. As a result of NI will have a mixed composition of the type MnO - SiO2 - Al2O3.