Abstract
Content
- Introduction
- 1. Formulation of research problems
- 2. Presentation of the material and results
- Conclusion
- References
Introduction
Among the products obtained by chemical synthesis, a significant share falls on methanol. Methanol a perfect substitute for fuels and raw materials for many industries of organic synthesis. Promising areas use of methanol include the: production acetic acid, wastewater treatment, synthetic protein, synthesis of hydrocarbons to produce fuel.
An important problem is to reduce the production cost of methanol and to strengthen environmental security by implementing of modern approaches. To effectively use the the advanced world experience in this area need to be developed within the framework of the national science solutions for learning, optimization and management of modern equipment and processes.
1. Formulation of research problems
In this paper we solve the problem of creating a conceptual and algorithmic foundations of diagnostic systems of water-cooled thermal reactor for methanol synthesis. The problem is solved by developing a methodology for the heat balance of the reactor and its use to determine the important process parameters during the design phase (maximum and nominal expenditures of steam and feed water) and create an algorithm for operative diagnosis the thermal work operation of the reactor by determining the share of spending the carbon monoxide in the synthesis and respectively, making judgments about the depletion the catalytic ability of the catalyst.
2. Presentation of the material and results
The scheme of water-cooled reactor for methanol synthesis with the proposed collection of items control instrumentation and automated process control system for the diagnosis of thermal work is presented in figure 1.
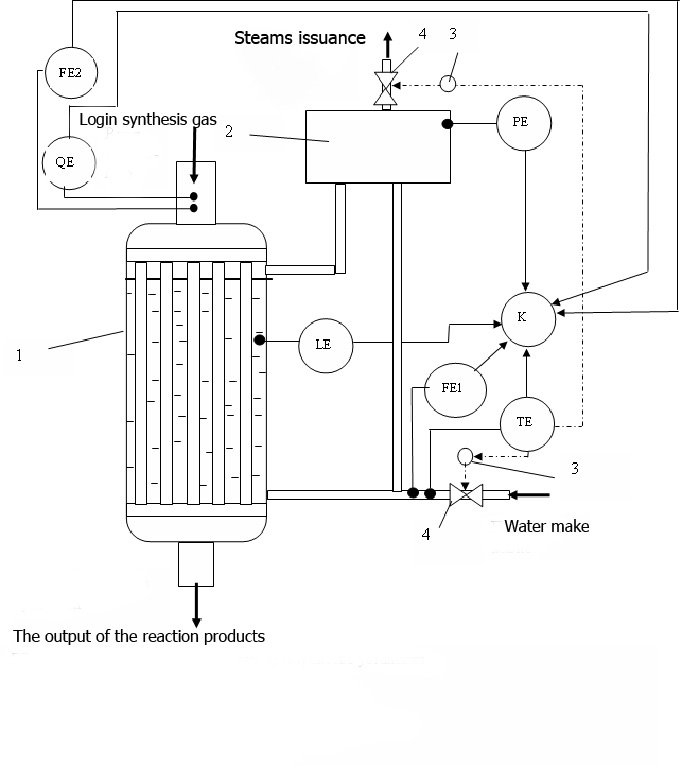
Figure 1 - Diagnostic systems the thermal work of the reactor for methanol synthesis
(1 - water cooled reactor, 2 - separator, 3 - executive mechanisms, 4 - regulators)
Diagnostics and analysis of the reactions exhaustion of the reactivity the catalyst asked to conduct based on an analysis of spending the carbon monoxide in the synthesis reactions, calculated depending on the current flow of feed water:
| ξ = | Gw · (iss − ifw) · 100 · 3600 · 22,4 | . |
| CO · V · Qm |
where ξ – share of spending the carbon monoxide in the synthesis reactions;
СО – the percentage of the carbon monoxide in the synthesis gas, %;
V – volumetric flow of synthesis gas, m3/h;
Qm – thermal effect of formation methanol, J/kmol;
Gw – water flow, kg/s;
iss, ifw – enthalpy of saturated steam at the outlet of the reactor and the feed-water, respectively, J/kg.
Availability of such information is necessary to make a decision on suspension process for the replacing or regenerating the catalyst. The accumulation of this information and analysis together with the statistics of other important changes in production factors can to deepen understanding of the process and find the conditions that allow to extend the time frame using the catalysts.
Creating conditions for sustainable use of modern equipment for methanol synthesis increases environmental safety by reducing the amount of processed raw materials, reduction of harmful emissions into the atmosphere and reducing the areas occupied by the respective companies.
Conclusion
The expediency of developing diagnostic systems of water-cooled the thermal work reactor for methanol synthesis, allowing make a judgment about the depletion of the reactivity the catalyst based on continuous analysis of the heat balance.
Proposed concrete scheme the thermal work diagnosis of water-cooled reactors for methanol synthesis and the algorithm for its use in the area of automated process control system of site of the methanol synthesis.
References
- Химическая технология ТГИ / Под ред. Г.Н. Макарова, Г.Д. Харламповича. – М.: Химия, 1986. – 496 с.
- Печуро Н.С. Химия и технология синтетического жидкого топлива и газа / Н.С. Печуро, В.Д. Капкин, О.Ю. Песин. – М.: Химия, 1986. – 352 с.
- Мещеряков Г.В. Химическая технология неорганических и органических веществ, теоретические основы / Г.В. Мещеряков // Химия и химическая технология .– 2009.– №6.– 86-88
- Ривкин С.Л. Термодинамические свойства воды и водяного пара. Справочник. - 2-е изд., перераб., и доп. / С.Л. Ривкин, А.А. Александров – М.: Энергоатомиздат, 1984. – 80 с.
- Теплотехника термической переработки твердых топлив: Учебное пособие/ Кравцов В.В., Бирюков А.Б., Дробышевская И.П. - Донецк: Издательство «Ноулидж», 2011. – 170с.
- Промышленные печи. Справочное руководство для расчетов и проектирования. 2-е издание, дополненное и переработанное. Казанцев Е.И. М., "Металлургия", 1975. 368 с.
- Бондалетова Л.И., Бондалетов В.Г. Процессы переработки сырья и рациональное использование природных ресурсов: Учебное пособие. – Томск: Изд-во ТПУ, 2006. – 160 с.
